60 6.2 Current and Potential Therapies for NSCLC
Current Therapies Used in Field of NSCLC
What Is Chemotherapy?
Chemotherapy is defined as a drug treatment that uses powerful chemicals to kill fast-growing cells within the human body (Zappa and Mousa). Decision to use chemotherapy is dependent on the cancer stage and other factors, such as growth within the body. It can be used in a variety of different situations:
- Neoadjuvant chemotherapy – Chemotherapy can be used before surgery to shrink the tumour before excision.
- Adjuvant chemotherapy – Chemotherapy can be used after surgery to kill cancer cells that were not removed with surgery. It can also be used to eliminate cancer cells spread to unknown areas within the body.
- Treating locally advanced NSCLC – If the patient is too weak for surgery, chemotherapy may be used to target cancer in the lungs, as well as the affected adjacent areas.
- Treating metastatic NSCLC – chemotherapy is used in extensive cases when cancer has spread outside of lungs
Video 1: Mechanism of cisplatin and how it is used to target cancer cells (URIanimation).
Types of chemotherapy drugs
Two of the most commonly used chemotherapy drugs are cisplatin and carboplatin. These drugs are not used in combination with one another, but can be combined with other drugs for a synergistic effect (Zappa and Mousa). Cisplatin causes cell death through binding to cancer cells and inhibiting DNA repair (Ho et al.). Carboplatin is known to form a reactive platinum complex that inhibits DNA synthesis; however, the exact mechanism is unknown (Ho et al.). Chemotherapy is administered intravenously for lung cancer.
Limitations of chemotherapy
Both these platinum based drugs have become essential in the gold standard treatment of malignancies. However, they can have side effects such as emesis (vomiting), anemia and toxic effects on many body systems, including the kidneys, brain, ear, and liver. These toxic effects can vary from patient to patient and are also dependent on the companion drugs used. Additionally, cancer cells can develop resistance to these drugs for many reasons, whether they are given at a low dose or a high dose. Finally, these chemotherapy drugs cannot distinguish healthy cells from tumour cells: they target all cells that are rapidly dividing, regardless of their function (Rossi and Di Maio).
What Is Radiation Therapy?
Radiation therapy is the process of using high-energy x-rays to kill cancer cells (Parashar et al.). Radiation is often combined with chemotherapy to target lung tumours that cannot be surgically treated or tumours that spread too quickly. Radiation therapy can be applied for 5 days a week for 5-7 weeks, and is often painless. There are two main types of radiation therapy (Parashar et al.) used for NSCLC:
- External beam radiation therapy (EBRT): Uses radiation beams from outside the body to target the cancer. This is used most often for NSCLC or cases where the cancer has metastasized to other organs.
- Brachytherapy (internal radiation therapy): This technique is used to shrink tumours in the airway to alleviate symptoms. Small sources of radioactive material are placed into the cancer with a bronchoscope.
Limitations of radiation therapy
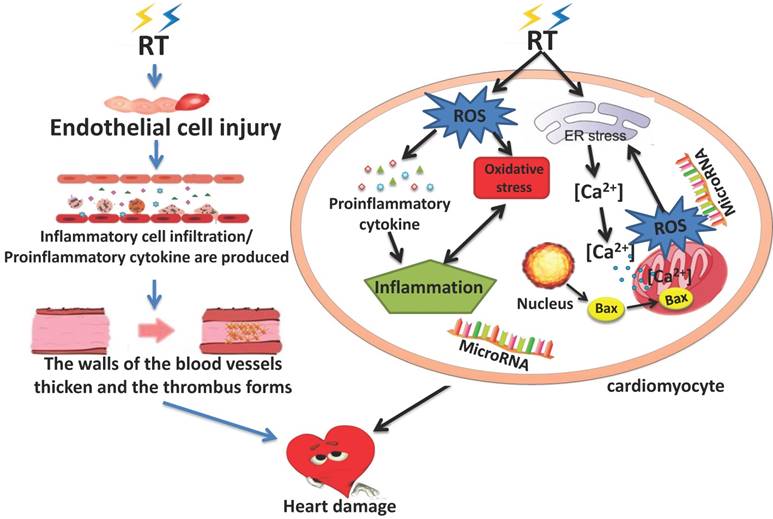
A serious side effect of radiation therapy is radiation-induced heart disease (RIHD). It is believed that RIHD is a result of damage to endothelial cells, inflammation, oxidative stress (OS), mitochondrial damage, endoplasmic reticulum stress, and micro-RNAs. RIHD can result in a variety of heart diseases including pericarditis, cardiomyopathy, coronary artery disease, valvular heart disease and cardiac conduction abnormality (Wang et al.).
What Is Targeted therapy?
Targeted therapy is a treatment that targets specific mechanisms used by NSCLC tumour cells to grow within the body (Yuan et al.). Targeted therapy drugs are administered orally therefore negating the need to go to a hospital for treatment. This is often an option for NSCLC when chemotherapy and radiation aren’t effective. It is also used to treat advanced stages of NSCLC. Two examples of how targeted therapy can be used (Yuan et al.):
- Targeting blood vessels: Targeted therapy can be used to target the tumour’s ability to create blood vessels, therefore keeping the tumour malnourished and causing tumour death. These drugs do not specifically target the tumour, but target its blood vessels instead.
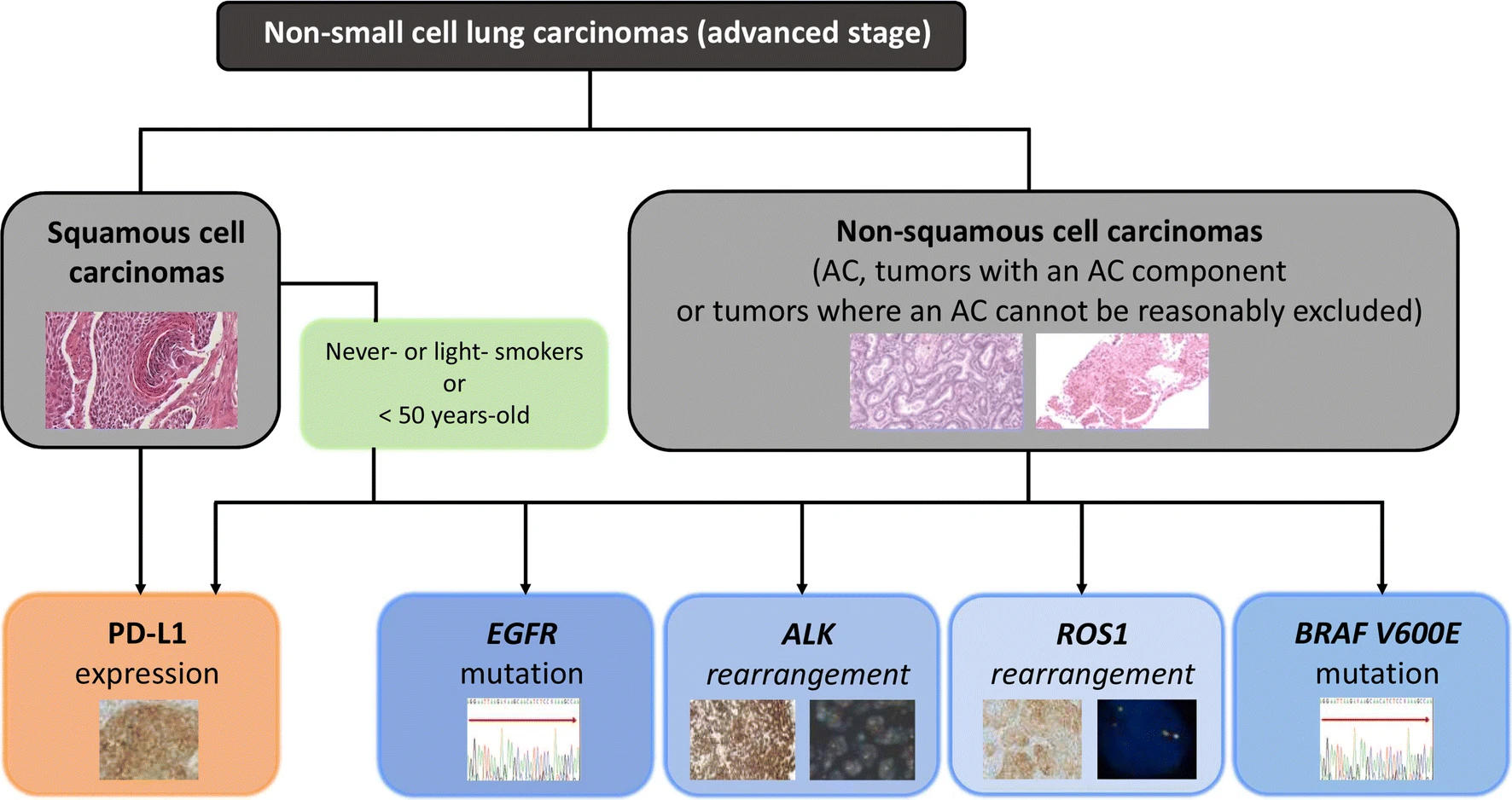
- Targeting oncogenic drivers: Targeted therapy can be used to inhibit signals or pathways involved in abnormal cellular proliferation and survival. One way it can do this is through tyrosine kinase inhibitors (TKI). For example, TKI can be used to target epidermal growth factor receptor (EGFR), a proto-oncogene. EGFR is expressed at high levels on NSCLC cells and, when mutated, drives tumour cell proliferation and survival. TKI binding to EGFR will lead to decreased tumour proliferation. Biomarker testing is used to identify the oncogenic drivers that can be targeted (Melosky et al.). In Canada, standard-of-care molecular testing for oncogenic drivers include EGFR, ALK, BRAF and ROS1 (Melosky et al.).
Limitations of targeted therapy
Since targeted therapy works by recognizing certain biomarkers, it is only effective for cancers that express those biomarkers. This treatment is ineffective on mutated cancers that no longer express wild type cancer biomarkers. Response to targeted therapy also varies from patient to patient, despite how some tumours have the same targeted mutations. Another limitation of using targeted therapy is resistance to treatment. Targeted therapy is used in order to prevent tumours from growing and spreading. Thus, combination therapy of targeted drugs with chemotherapy and radiation therapy is often used, which can also help prevent resistance (Lee et al.).
What Is Immunotherapy?
Immunotherapy is a treatment that assists the immune system in targeting and destroying cancers. The immune system uses certain checkpoints to ensure that it does not attack the healthy self-cells. Tumour cells use these same checkpoints to persist within the body (Doroshow et al.). One of such checkpoints is the interaction between the programmed death 1 (PD-1) protein, which is expressed on the surface of T cells, and its ligand, program death ligand 1 (PD-L1). When these two bind, it will initiate a suppressive effect on T cells, therefore inhibiting the T cell activation (Doroshow et al.). In healthy individuals, PD-L1 is expressed by self-cells to ensure normal cells are not being attacked. However, tumours have manipulated this mechanism by expressing PD-L1 on their surface as well, allowing it to evade the immune system. Immunotherapy drugs have been developed to inhibit this interaction by either binding to PD-L1 on tumour cells or PD-1 on T cells (Doroshow et al.).
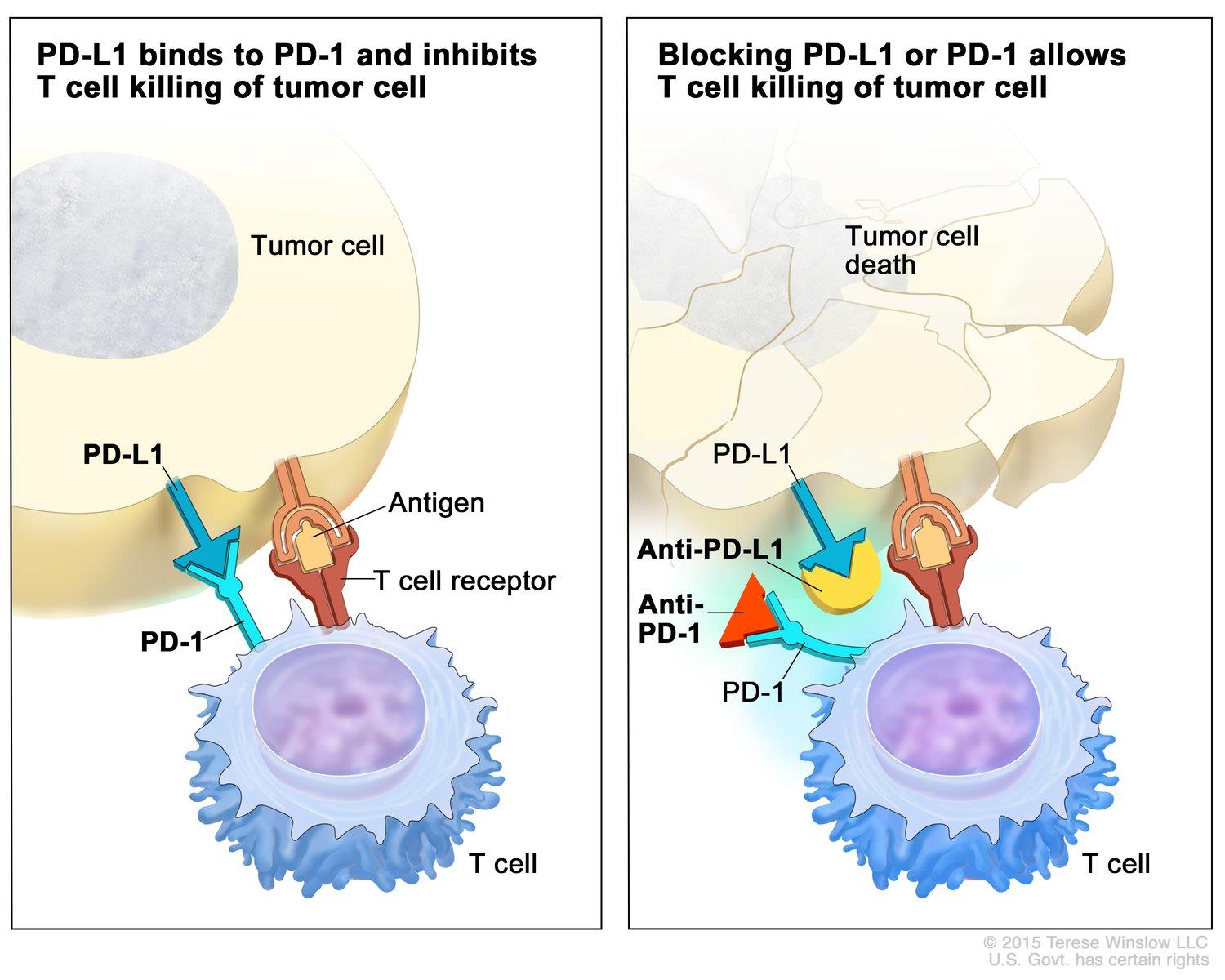
Another drug aimed at inhibiting checkpoint inhibitors involves the CTLA-4 protein. CTLA-4, expressed on T cells, binds to B7 on other cells to inhibit T cell activation (Puri and Shafique). Blocking CTLA-4 can prevent T cell inactivation and promote tumour cell killing. Anti-CTLA-4 drugs are usually used in combination with drugs targeting PD-L1 (Puri and Shafique).
Limitations of immunotherapy
Immunotherapy has become a promising approach in cancer treatment, however, it is limited to certain cancers due to the expression levels of PD-1 in the tumours. Additionally, there are PD-L1-expressing tumours that do not seem to respond to PD-1/PD-L1 inhibitors. Compared to the toxicities caused by chemotherapy and radiation therapy, side effects of immunotherapy are less severe and are mostly seen with longer treatment duration (Alsaab et al.). Another limitation of immunotherapy is the development of autoimmune effects, this is where the immune system will begin to target tissues in the body (Leighl).
A New Potential Therapy For NSCLC: CAR T Cell Therapy with PD-L1
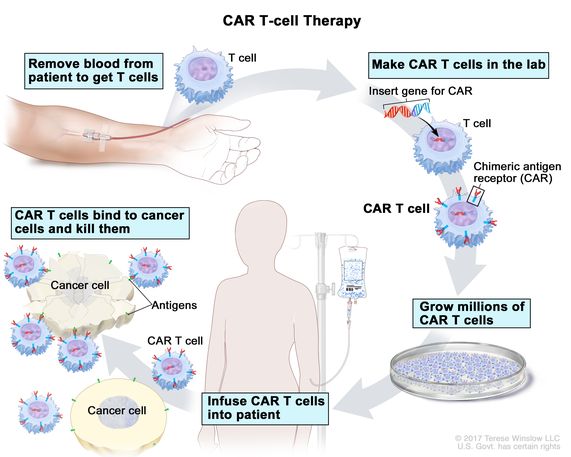
Chimeric antigen receptors (CAR) T cell therapy is a novel method diverging from traditional immunotherapy methods where it includes the use of artificially created T cell receptors that have engineered specificity. T cells from patients are extracted from blood and genetically engineered to produce receptors on their surface called chimeric antigen receptors (Liu et al.). This allows T cells to recognize and attach to specific antigens on tumour cells, providing specific cytotoxic effects towards tumour cells. The field of immunotherapy is currently looking at using these CAR T cells to target PD-L1 on tumour cells, thus inhibiting the interaction between PD-L1 and PD-1, which downregulates T cells (Liu et al.). This novel treatment gives a more host-specific treatment as the process involves the patients’ own immune cells to treat cancer. Mechanisms, efficacy, and limitations of CAR T cells will be discussed in the next two section of this chapter.
References
Alsaab, Hashem O., et al. “PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome.” Frontiers in Pharmacology, vol. 8, 23 Aug. 2017, doi:10.3389/fphar.2017.00561.
Diagnostic Algorithm for Biomarker Testing in Patients with Advanced NSCLC. 1 July 2020. link.springer.com, https://link.springer.com/article/10.1007/s12094-019-02218-4.
Doroshow, Deborah B., et al. “Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes.” Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, vol. 25, no. 15, 01 2019, pp. 4592–602. PubMed, doi:10.1158/1078-0432.CCR-18-1538.
Ho, Gwo Yaw, et al. “Cisplatin versus Carboplatin: Comparative Review of Therapeutic Management in Solid Malignancies.” Critical Reviews in Oncology/Hematology, vol. 102, June 2016, pp. 37–46. PubMed, doi:10.1016/j.critrevonc.2016.03.014.
Lee, Yeuan Ting, et al. “Molecular Targeted Therapy: Treating Cancer with Specificity.” European Journal of Pharmacology, vol. 834, 5 Sept. 2018, pp. 188–196., doi:10.1016/j.ejphar.2018.07.034.
Leighl, Natasha. Expert’s Corner: Lung Cancer. Interview by Janice Li, 5 Dec. 2020, https://play.library.utoronto.ca/play/4a3ae2fc17a78d551d4cd9404e52bc66.
Liu, Ming, et al. “Targeting PD-L1 in Non-Small Cell Lung Cancer Using CAR T Cells.” Oncogenesis, vol. 9, no. 8, 8, Nature Publishing Group, Aug. 2020, pp. 1–11. www.nature.com, doi:10.1038/s41389-020-00257-z.
Melosky, B., et al. “Standardizing Biomarker Testing for Canadian Patients with Advanced Lung Cancer.” Current Oncology, vol. 25, no. 1, 1, Feb. 2018, pp. 73–82. www.current-oncology.com, doi:10.3747/co.25.3867.
National Cancer Institute. Immune Checkpoint Inhibitors. 24 Sept. 2019, https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors.
Parashar, Bhupesh, et al. “Radiation Therapy for Early Stage Lung Cancer.” Seminars in Interventional Radiology, vol. 30, no. 2, June 2013, pp. 185–90. PubMed, doi:10.1055/s-0033-1342960.
Puri, Sonam, and Michael Shafique. “Combination Checkpoint Inhibitors for Treatment of Non-Small-Cell Lung Cancer: An Update on Dual Anti-CTLA-4 and Anti-PD-1/PD-L1 Therapies.” Drugs in Context, vol. 9, 2020. PubMed, doi:10.7573/dic.2019-9-2.
Rossi, Antonio, and Massimo Di Maio. “Platinum-Based Chemotherapy in Advanced Non-Small-Cell Lung Cancer: Optimal Number of Treatment Cycles.” Expert Review of Anticancer Therapy, vol. 16, no. 6, 8 Jan. 2016, pp. 653–660., doi:10.1586/14737140.2016.1170596.
URIanimation. The Mechanism of Cisplatin (New -HD). 2009. YouTube, https://www.youtube.com/watch?v=Wq_up2uQRDo.
Wang, Heru, et al. “Radiation-Induced Heart Disease: a Review of Classification, Mechanism and Prevention.” International Journal of Biological Sciences, vol. 15, no. 10, 8 Aug. 2019, pp. 2128–2138., doi:10.7150/ijbs.35460.
Yuan, Min, et al. “The Emerging Treatment Landscape of Targeted Therapy in Non-Small-Cell Lung Cancer.” Signal Transduction and Targeted Therapy, vol. 4, no. 1, 1, Nature Publishing Group, Dec. 2019, pp. 1–14. www.nature.com, doi:10.1038/s41392-019-0099-9.
Zappa, Cecilia, and Shaker A. Mousa. “Non-Small Cell Lung Cancer: Current Treatment and Future Advances.” Translational Lung Cancer Research, vol. 5, no. 3, June 2016, pp. 288–300. PubMed, doi:10.21037/tlcr.2016.06.07.
