52 4.5: ELISA
The enzyme-linked immunosorbent assay is an analytical biochemistry assay created in 1971 by Swedish scientists, Eva Engvall and Peter Perlman (Aydin, 4). This was a really important invention and has since been an indispensable method used for research. ELISA is used to measure antibodies, antigens, proteins and glycoproteins in biological samples. It uses antibodies for detection against the protein that is to be measured.
How it works
ELISA is mainly known for its ability to immobilize reagents to the microplate surface. It also has the ability to utilize high affinity antibodies while washing away non-specific materials. There are different variations of ELISA but most follow the same basic structure: coating, plate blocking, detection, and signal measurement.
- Coating: Antigens are immobilized to the microplate surface
- Plate blocking: Protein are added to cover unsaturated surface binding sites of microplate
- Detection: Microplate is incubated with high-affinity antibodies binding to the antigens
- Signal measurement: Signal is generated and detected by the direct or secondary tag of the antibody
ELISA and LIF
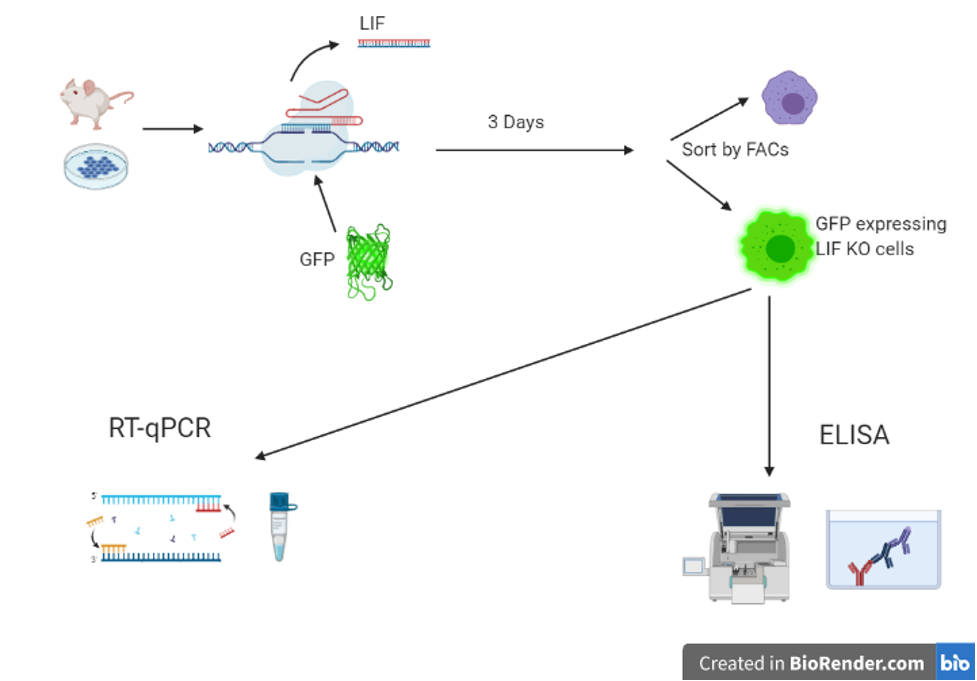
ELISA remains as a crucial experimental technique for researchers. Referring back to the Pascual-García, et al. study, ELISA was used to study mice that were transfected with LIF. A green fluorescent protein (GFP) was used as a selective label. The cells were then analyzed by ELISA. The cells with the LIF knockout displayed higher levels of CXCL9. Figure 1 highlights the experimental process.
Test your knowledge:
