29 3.8 Melittin Synergistically Improves Docetaxel Treatment and Reduces Immune Suppression In Vivo
Overview
The ability of Honey Bee Venom to improve the anticancer potency of current oncological therapies has been previously demonstrated in vitro, through combination therapy strategies on Cervical Cancer cell lines (Gajski et al. 2013). This chapter will explain how researchers have expanded the scope of these results to in vivo models of Triple-Negative Breast Cancer (TNBC), and the important role that Melittin may play in sensitizing Breast Cancer tumors to Docetaxel therapy, as well as reducing the immune-suppressive effects of TNBC tumors.
An In-vivo Mouse Model for Aggressive Triple-Negative Breast Cancer
BALB/cJ female mice were chosen as models for Triple-Negative Breast Cancer (TNBC). An important feature of these mice is their intact immune system, which allows for a more authentic and reliable model of tumor progression and immune response to the experimental treatment regimes. Murine p53- T11 TNBC was the choice cell line, and these cells were transplanted into the flanks of the BALB/cJ mice to form the final mouse allograft of aggressive TNBC.

A brief visual summary of the generation of in-vivo mice models of aggressive TNBC with immune competency. © BioRender.
Melittin Sensitizes Triple-Negative Breast Cancer Cells to Docetaxel Treatment
Docetaxel is a common cytotoxic Breast Cancer therapeutic that broadly functions to stabilize Microtubule assembly and disrupt cellular division. When employed in a combination therapy with Melittin, Docetaxel was able to significantly reduce TNBC tumor growth in mice models when compared with the efficacy of both treatments applied individually. This result bears implications that Melittin may be able to improve the efficacy of current treatments targeting breast cancer tumors.
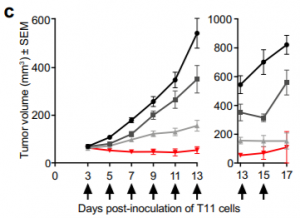

A graphical representation of the vastly reduced tumor growth exhibited in TNBC mice treated with the Melittin + Docetaxel combo compared to the other treatment protocols (Duffy et al. 2020).
Concept and Methodology Review
PD-L1 is an immune-checkpoint transmembrane protein expressed on cells to contact the PD-1 receptor on T-cells, inhibiting T-cell immune functions such as induction of apoptosis and cytokine release. PD-L1 may be expressed by cancerous cells to inhibit the T-cell immune response.
Immunohistochemistry and Immunofluorescence staining techniques were employed to validate the positive anticancer effects observed in the combination therapy. The Ki-67 assay indicated a marked reduction in tumor proliferation and the TUNEL assay displayed enhanced DNA fragmentation and tumor cell apoptosis in the mice tissue treated with the Melittin + Docetaxel combination therapy.
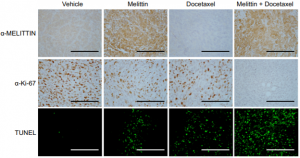
α-Ki67 assay and TUNEL immunofluorescence exhibiting reduced tumor proliferation and enhanced cancer cell apoptosis in mice models treated with the Melittin + Docetaxel combination therapy (Duffy et al., 2020).
Melittin Reduces the Population of Immune-Evasive PD-L1 Positive Cancer Cells
Melittin therapy individually and in combination with Docetaxel significantly reduced the populations of cancer cells expressing the PD-L1 ligand. With fewer cells expressing PD-L1, T-cell immune function is partially restored to mediate their anticancer effects at the tumor site. Melittin may be able to reduce the immune-suppressive effects of the TNBC microenvironment in order to boost the immune system’s ability to fight off the cancer. Immunostaining for the PD-L1 ligand shows that it’s expression is significantly reduced in the tumor tissues of mice treated with Melittin and Melittin + Docetaxel.
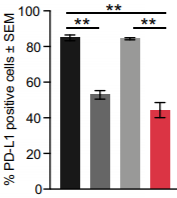

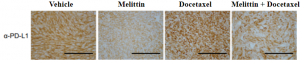
PD-L1 positive TNBC cell population was significantly reduced in both Melittin and Melittin + Docetaxel combo. This result was confirmed by α-PD-L1 assay staining for PD-L1 that was less expressed in Melittin treated mice tumors (Duffy et al. 2020).
- In vivo experiments were performed on mice models with intact immune systems and transplanted with cancerous cells
- Melittin combination therapy was capable of increasing the anticancer activity of the common breast cancer chemotherapeutic agent Docetaxel
- Melittin individually and in combination with Docetaxel was capable of reducing the population of tumor cells overexpressing the PD-L1 immune-checkpoint ligand, effectively reducing the T-cell suppression of the TNBC tumor environment
References
- Gajski G., Čimbora-Zovko T., Rak S., Rožman M., Osmak M., & Garaj-Vrhovac V. (2013). Combined antitumor effects of bee venom and cisplatin on human cervical and laryngeal carcinoma cells and their drug resistant sublines. Journal of Applied Toxicology, 34(12), 1332-1341. doi:10.1002/jat.2959
