1 1.1 Overview of COPD and Asthma
What is COPD?
Chronic obstructive pulmonary disorder (COPD) is a progressive disease that is defined by loss of airflow due to airway obstruction. The air sacs of the lungs lose their elasticity, and the walls are destroyed, inflamed, and clogged with mucus. COPD is acknowledged as an umbrella term that encompasses two conditions: chronic bronchitis and emphysema (Dai et al., 2017). The range of symptoms from each of these conditions varies per individual, posing a daily burden and reducing the health-related quality of life. The Global Burden of Disease Study conducted in 2015 showed an estimate of 174 million cases of COPD globally (Vos T. et al 2015). The risk of developing COPD, however, differs from country to country, with the highest prevalence in the United States. There has also been a significant increase between 1990 and 2010 in the Eastern Mediterranean and African regions (Adeloye D. 2015). Diagnosis for COPD is most prevalent in older adults and is associated with high morbidity, being the third leading cause of mortality worldwide (Leather et al., 2019).
Causes and Symptoms
The main cause of COPD is long-term exposure to irritants that damage the lungs and its airways. The main and most major risk factor is smoking and exposure to tobacco through inhalation, as well as through second-hand smoke. Other common risk factors for COPD include air pollution, inhalation of noxious particles, pre-existing respiratory conditions and infections, and low socioeconomic status (Tarzikhan et al., 2016). Although rare, a genetic condition termed alpha-1 antitrypsin deficiency can put an individual at greater risk for lung-related diseases such as COPD and cause related emphysema. It is characterized by low levels of alpha-1 antitrypsin, a protease inhibitor that is secreted by hepatocytes serves a protective function for lung tissue (Torres-Duran et al., 2018). COPD is associated with a range of symptoms that vary in severity and pose burdens and challenges on an individual. The most common symptoms include shortness of breath, coughing, and sputum production. Other bothersome symptoms that can impact daily life are chest tightness, congestion, and wheezing (Miravitlles and Ribera, 2017). These symptoms may begin as mild and become severe over time with more exposure. The evaluation of these symptoms is important for diagnosis and measured through techniques such as spirometry and validated patient questionnaires. COPD specific markers can also be observed such as post-bronchodilator response, which measures the forced expiratory volume over the forced vital capacity. If this measurement is less than 70%, it may be concluded that an individual could have COPD. Other markers include airway neutrophils and overall declined lung function. Please see the video below from the NHLBI (National Heart, Lung, and Blood Institute) to learn more about the diagnosis of COPD.
Definitions
Spirometry: a diagnostic test used for respiratory conditions to determine pulmonary function, measuring the amount of speed and air that an individual can inhale and exhale.
Forced expiratory volume (FEV): the amount of air an individual can exhale during a forced breath.
Forced vital capacity (FVC): the amount of air that can be exhaled forcibly after taking the deepest breath possibile.
Pathogenesis
There are several mechanisms that are involved in the progression of COPD that lead to destruction of airways, inflammation and loss of lung tissue as well as the disorganization of alveoli. The pathogenesis of COPD evolves as the disease progresses, leading to health deterioration and lower quality of life. It is characterized by an increase in dendritic cells, macrophages and neutrophils are recruited to the airway epithelium in response to pollutants and oxidants. Macrophages play a role in tissue maintenance, producing proteolytic enzymes and strengthening the release of TNF-alpha (tumor necrosis factor) and IL-8 (interleukin). Other inflammatory cells such as CD4+ and CD8+ T-cells are recruited and responsible for the release of chemokines. CD8+ cytotoxic T-cells are predominant responsible for killing infected and damaged cells, and mediating inflammation. However, they are more prominent in smokers and can also play a role in the development of emphysema through apoptosis of lung cells. (Bagdonas et al., 2015). This inflammation and pathogenic process can lead to airflow limitation, hypersecretion of mucus, small airway fibrosis, obstructive bronchiolitis and emphysema. Exposure to cigarette smoke and environmental toxins can also induce oxidative stress and create oxidative injury through creation of reactive oxygen species (ROS). This causes altered DNA damage and repair processes and DNA strand breaks which can lead to lower cell proliferation, increased apoptosis and necrosis (Caramori et al., 2016)
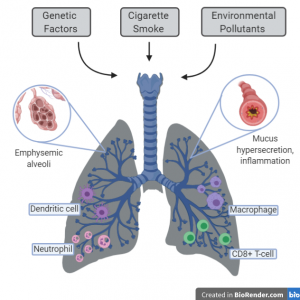
Figure 1. The immune cells and mechanisms involved in COPD development.
What is Asthma?
Asthma is one of the most common respiratory diseases worldwide and the number of individuals that suffer from this are predicted to reach 300 million by the year 2025. Although it does not appear as a major cause of death, it remains in the top 50 causes of mortality and causes of death. Asthma is a heterogenous inflammatory disease that effects the lower airway of the lungs and can be triggered by susceptibility to viral infections, allergens, smoke exposure and air pollution. As opposed to COPD, it affects all ages but is the most prevalent in children between 5 and 14 years (Fehrenbach et al., 2017). The symptoms for asthma are prevalent in both low and high-income countries and are more severe for children in low and middle-income (Frati et al., 2019). The current perception of asthma encompasses multiple phenotypes. For example, asthma in adulthood is considered as a syndrome with the common characteristic of airflow limitation. In general, in is classified into atopic (allergic) or nonatopic (nonallergic) and distinguished by the IgE antibody response.
Causes and Symptoms
There is no single cause for asthma, and it has believed to be caused by multiple genetic and environmental factors with symptoms varying per individual. All age groups can be affected but most frequently develops at childhood. The genetic cause of asthma is recognized by multiple single nucleotide polymorphisms (SNPs) and it has been shown that the children of parents with asthma are more likely to suffer from it (Thibeault and Laprise., 2019). However, the interaction of genes and the environment as well as epigenetic changes play a larger role in asthma development and responses to treatment. The most common allergens from the environment include dust, grass, pollen, fungi, and mold (Frati et al., 2019). Inflammation of the airways caused by asthma can limit airflow and produce symptoms such as wheezing, dyspnea, chest tightness, and cough that can vary in intensity. Allergic asthma is early onset and associated with the triggering of allergens leading to an increase in IgE antibodies. In contrast, nonallergic asthma is late-onset and depicts no genetic or hereditary patterns, with no serum IgE levels detected. Multiple tests are done to diagnose asthma patients such as skin prick tests, spirometry, bronchodilator test, and blood tests for detection of eosinophils. Other biomarkers for detection of asthma can include endotypes for T-helper cells and exhaled nitric oxide (FeNO), as seen in the diagram below (Loureiro et al., 2018).
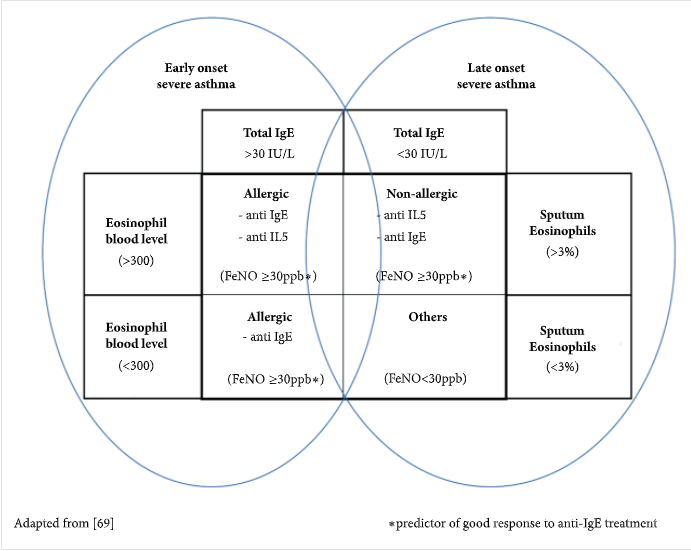
Figure 2. Proposed biomarkers and values to diagnose severe asthma.
Definitions
Single nucleotide polymorphism (SNP): the substitution of a nucleotide at a specific position of the genome.
Dyspnea: the medical term for shortness of breath.
Pathogenesis
Asthma is mainly characterized by the activation of mast cells and infiltration of eosinophils. Unlike COPD, this is driven by type II T-helper cells and innate lymphoid cells (Rogliani et al., 2016). Dendritic cells and basophils are responsible for taking up the allergens and presenting them to T-cells. The differentiation of T-helper cells secretes cytokines such as IL-4 (interleukin), IL-5 and IL-13, which activate mast cells and recruitment of eosinophils. Eosinophils are responsible for remodeling airways, heightening the immune response, and activation of inflammation. Mast cells release granules that act on neurons and induce constriction of the airways and signal goblet cells to produce and secrete mucus. The humoral response is activated and causes the secretion of IgE antibodies. The binding of the allergen to the IgE receptor on basophils and mast cells causes the secretion of mediators of inflammation. These mechanisms create long-term asthmatic airway remodeling that is characterized by inflammation and basement membrane thickening, mucus hypersecretion, smooth muscle hypertrophy, and fibrosis (Thibeault and Laprise., 2019).
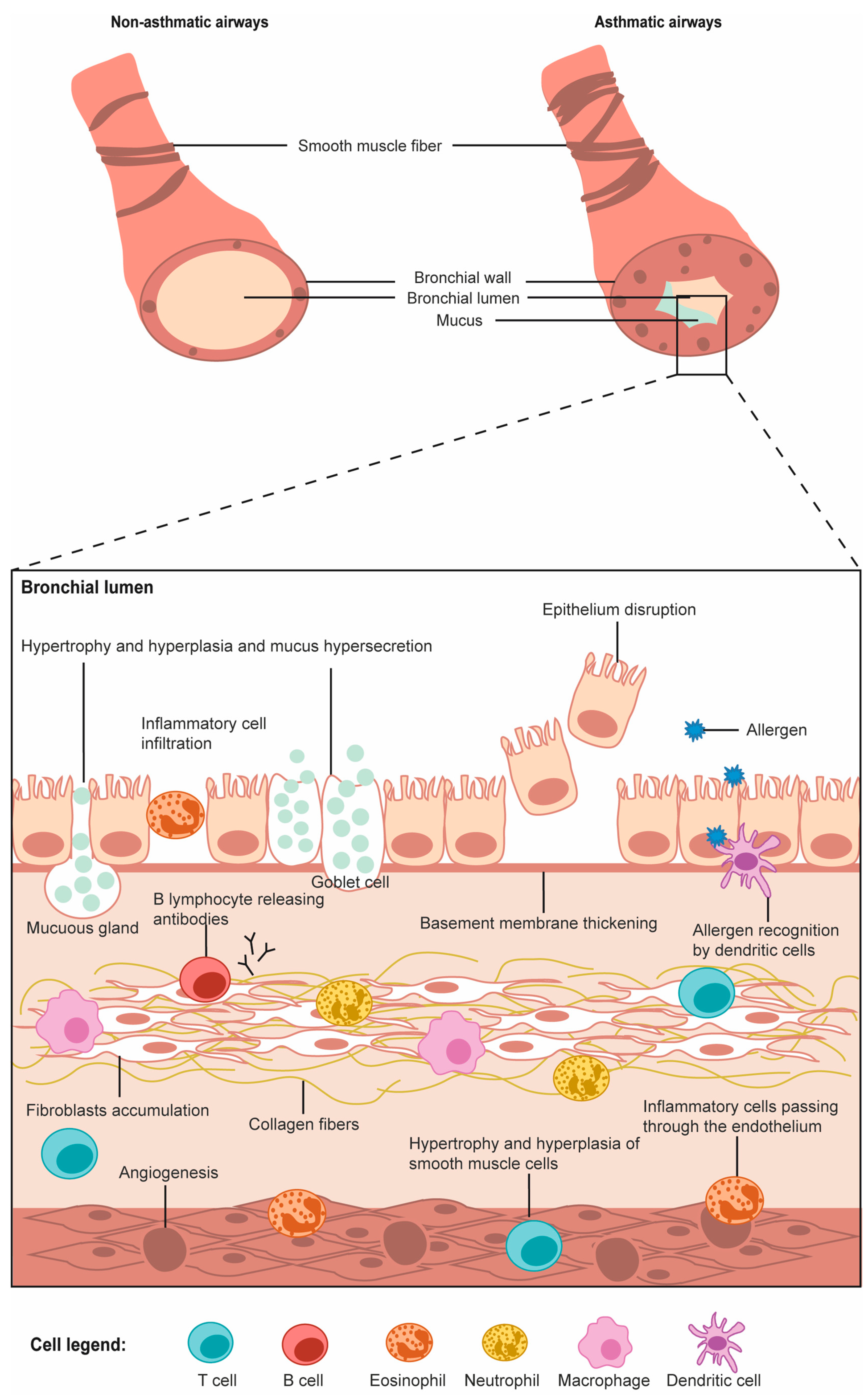
Figure 3. Schematic of the respiratory and immune cells involved in asthma (Thibeault and Laprise, 2019).
What is ACO?
Asthma-chronic obstructive pulmonary disease (ACO) describes a COPD and asthma overlap and is the focus of this chapter. It has a prevalence rate of 15-20% in populations with respiratory diseases, and patients diagnosed with ACO have similar but far more intense symptoms and pathogenesis leading to a greater socioeconomic burden compared to having asthma or COPD individually (Ishiura Y., 2019).
References:
1. Ishiura Y., et al. Effect of triple therapy in patients with asthma-COPD overlap. 2019. Int J Clin Pharmacol Ther. 384-392.
2. Veo T., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015.
3. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. 2015. J Glob Health. 5: 020415.
4. Rabe K., Watz H. Chronic obstructive pulmonary disease. 2017. The Lancet. 1931-40.
5. Dai J, Yang P, Cox A, Jiang G. Lung cancer and chronic obstructive pulmonary disease: From a clinical perspective. 2017. Oncotarget. 8(11):18513-18524.
6. Leather DA, Yates L, Svedsater H, Jacques L, Collier S, Powell D, Jones R. Can medicines development improve outcomes in asthma and chronic obstructive pulmonary disease management by driving effectiveness? 2019. Respir Res. 20(1):173.
7. Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. 2017. Respir Res. 2017;18(1):67.
8. Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and non-smokers: the Rotterdam Study. 2016. Eur J Epidemiol. 31(8):785-792.
9. Torres-Durán M, Lopez-Campos JL, Barrecheguren M, et al. Alpha-1 antitrypsin deficiency: outstanding questions and future directions. 2018. Orphanet J Rare Dis. 13(1):114.
10. Bagdonas E, Raudoniute J, Bruzauskaite I, Aldonyte R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. 2015. Int J Chron Obstruct Pulmon Dis. 10:995-1013.
11. Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, Adcock I. COPD immunopathology. 2016. Semin Immunopathol. 38(4):497-515.
12. Rogliani P, Ora J, Puxeddu E, Cazzola M. Airflow obstruction: is it asthma or is it COPD?. 2016. Int J Chron Obstruct Pulmon Dis. 11:3007-3013.
13. Frati F, Salvatori C, Incorvaia C, et al. The Role of the Microbiome in Asthma: The Gut⁻Lung Axis. 2018. Int J Mol Sci. 20(1):123.
14. Loureiro CC, Amaral L, Ferreira JA, et al. Omalizumab for Severe Asthma: Beyond Allergic Asthma. 2018. Biomed Res Int. 2018:3254094.
15. Hudon Thibeault, Andrée-Anne, and Catherine Laprise. “Cell-Specific DNA Methylation Signatures in Asthma.” Genes, vol. 10, no. 11, Nov. 2019, p. 932. www.mdpi.com, doi:10.3390/genes10110932.
