14 4.1. Overview of Alzheimer’s Disease
Learning Objectives
- Understand the difference between Dementia and Alzheimer’s disease
- Understand the two key diagnostic methods used to clinically detect Alzheimer’s disease
- Distinguish between CDR scores
- Understand the current treatment options available for mild, moderate, and severe Alzheimer’s
- Understand the Amyloid Hypothesis
- Distinguish between the two major pathologies of Alzheimer’s disease: plaques and neurofibrillary tangles
- Understand the history behind Alzheimer’s disease
- Gain insight on the importance of amyloid-β and the mutations associated with its link to Alzheimer’s
The Basics and Prevalence of Alzheimer’s Disease
Dementia is a syndrome that is clinically characterized by the progressive decline in at least two distinct cognitive domains. These include memory, language, executive and visuospatial function, personality, and behavior. Thus, impairing an individual’s ability to perform instrumental and/or basic activities of daily living. Alzheimer’s disease (AD) is one of the most common causes of dementia, accounting for roughly 80% of all dementia diagnoses. Whilst dementia is a general term used to describe a decline in mental ability (severe enough to interfere with daily life), Alzheimer’s is a specific disease. Alzheimer’s may also be split into three different types: early-onset, late-onset, and familial.
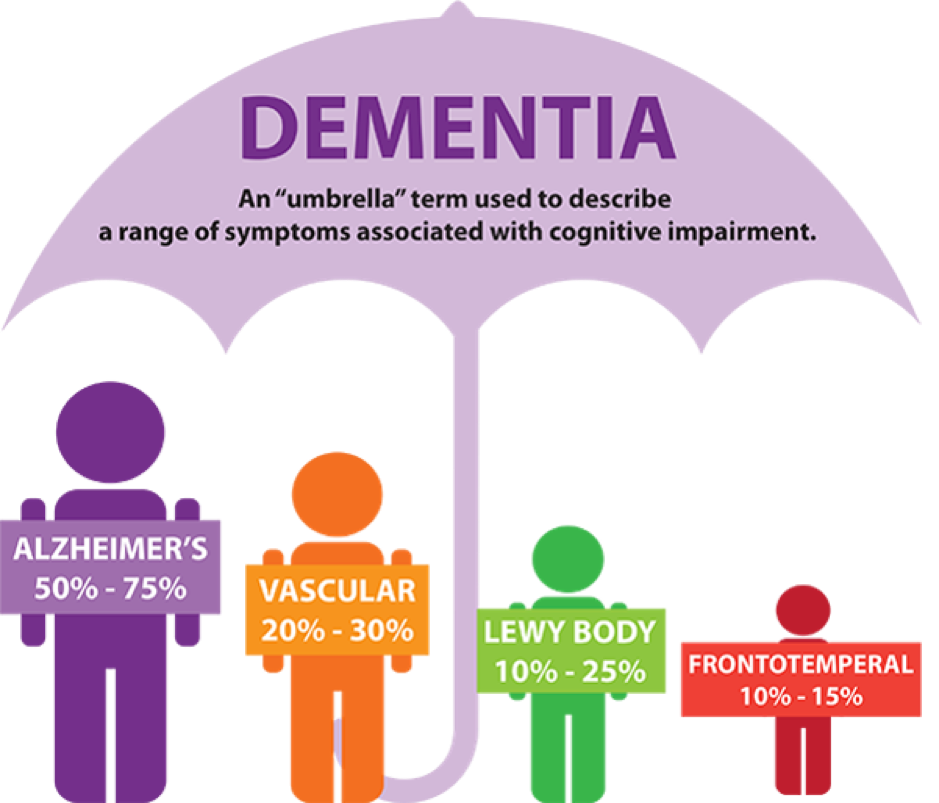
Based on national statistics, there are roughly 747,000 Canadians living with Alzheimer’s or a different type of dementia. Whilst globally, there are around 44 million people living with this disease, which is more than the total population of Canada. The true number is said to be much higher, as the disease starts developing in the brain around two to three decades before the onset of symptoms. Due to the increased prevalence, the mortality rate is also rising, increasing by roughly 89% between 2000 and 2014 in the United States.

How Clinicians Diagnose Alzheimer’s Disease
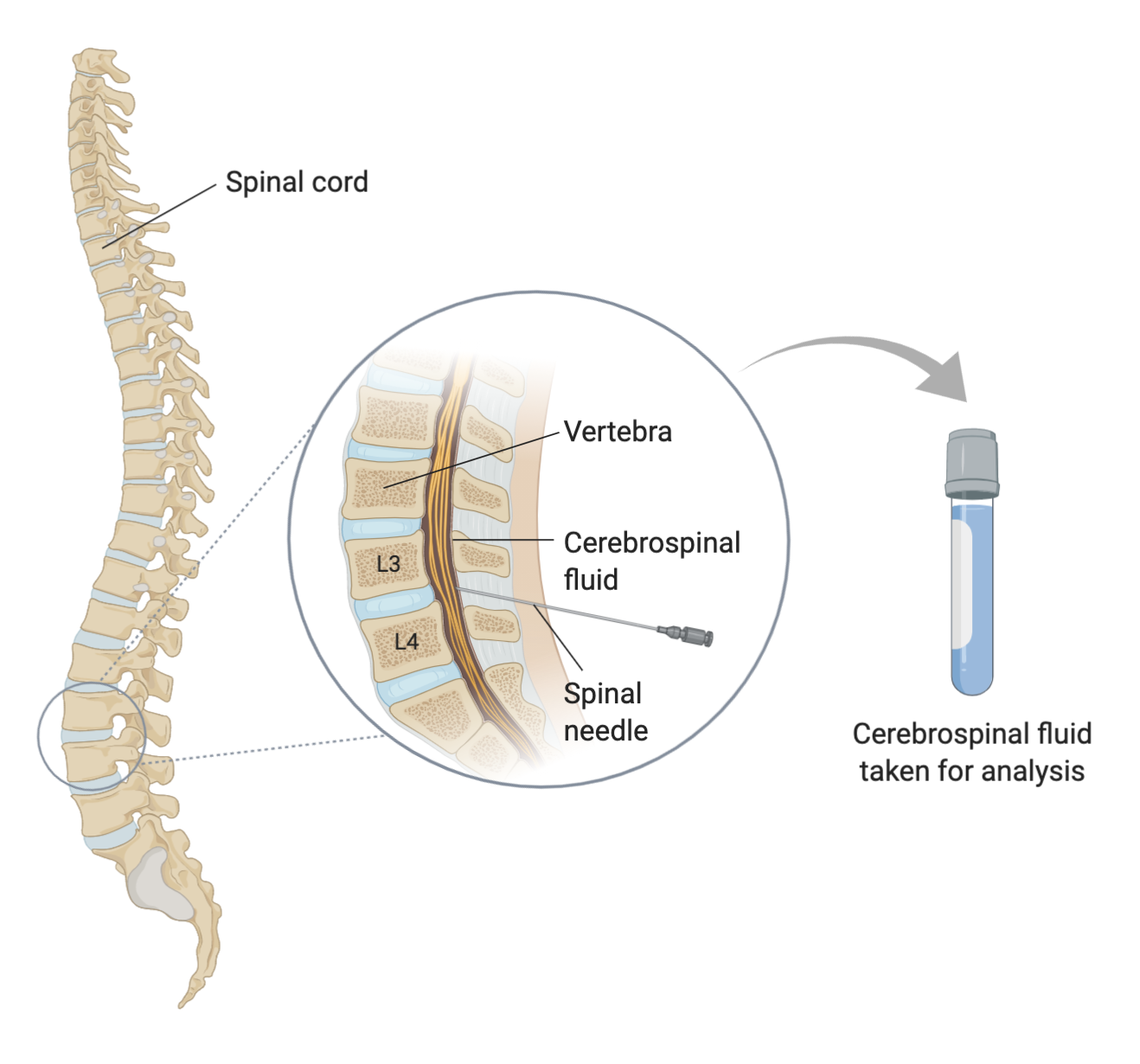
There are two prominent techniques that allow for scientists to detect Alzheimer’s, which include analyzing cerebrospinal fluid (CSF) for tau peptide and amyloid biomarkers and using Positron Emission Tomography (PET) to view levels of the mentioned protein deposits. CSF analysis is a more-invasive but less-costly examination, which involves the analysis of CSF biomarkers for Alzheimer’s. The technique does not have the greatest diagnostic accuracy, carries the risks associated with a lumbar puncture procedure, and oftentimes takes a while to obtain results due to lengthy fluid analysis. On the other hand, PET was revolutionary in the world of medicine, as the creation of a non-invasive diagnostic imaging method helped researchers make remarkable progress in the diagnostic accuracy of Alzheimer’s. To perform PET imaging, the patient is first injected with a radiolabeled tracer agent and then scanned using the PET scanner, which detects plaques formed by amyloid-β (Aβ) and the deposition of tau-protein in the brain.

Clinical Dementia Rating Scores
The Clinical Dementia Rating (CDR) was first introduced in 1982 and has since been used globally as a tool for grouping individuals with mild to moderate dementia. CDR scores are given as an estimate by a clinician during an interview with both the patient and their primary caregiver. The interview consists of questions relating to six distinct cognitive and behavioral domains, which include: memory, orientation, judgment and problem solving, community affairs, home and hobbies performance, and personal care. A CDR is scored on a scale of 0–3: no dementia (CDR = 0), questionable dementia (CDR = 0.5), mild cognitive impairment (CDR = 1), moderate cognitive impairment (CDR = 2), and severe cognitive impairment (CDR = 3). The questions are split into two sets, the first being for the patient, and the second for their caregiver. The questions for the caregiver consist of information regarding the patient’s memory, judgment, problem-solving ability, community affairs, home life, and hobbies. While, the questions for the patient consist of topics in relation to their memory, orientation, judgment, and problem-solving skills.
Current Treatments
The current treatment for mild to moderate Alzheimer’s disease is the use of cholinesterase inhibitors, which block the normal breakdown of acetylcholine. This neurotransmitter is present in both the Peripheral and Central Nervous Systems, and functions to enhance communication between nerve cells. In an individual with Alzheimer’s disease, there is a decrease in the levels of acetylcholine, leading to poor cellular communication. Cholinesterase inhibitors block the actions of an enzyme known as acetylcholinesterase from breaking down acetylcholine. Thus, increasing the concentration of acetylcholine in the brain and enhancing nerve cell communication.
For moderate to severe Alzheimer’s the current treatment is a medication called memantine, which works as an NMDA receptor inhibitor. The medication blocks the NMDA glutamate receptors, as the excessive stimulation of glutamatergic signaling may result in excitotoxicity. The excitotoxicity has been linked with neurodegeneration and is mediated by an increased Ca2+ entry through NMDA receptors. As well, excessive levels of glutamate may cause neurotoxicity, which may disrupt or kill nerve cells in the brain.
As both treatments, cholinesterase inhibitors and NMDA antagonists work in different ways, the two treatment options may be combined and used concurrently.

The Amyloid Hypothesis
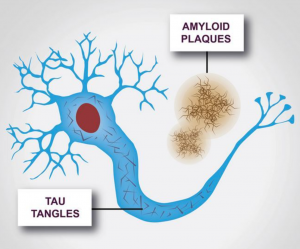
Alzheimer’s disease is characterized by two major pathologies: clumps of amyloid-β, which are known as plaques, forming outside of cells, and strings of a protein called tau, also known as neurofibrillary tangles, forming inside of cells. The amyloid hypothesis states that the accumulation of amyloid-β peptides in the brain is the driving force behind Alzheimer’s disease pathogenesis. The remainder of the disease process, which involves the formation of neurofibrillary tangles, is believed to originate from an imbalance between amyloid-β production and clearance. Scientists believe that the aforementioned pathologies play a key role in preventing communication between nerve cells, and disturbing all processes required for cell survival. In particular, it is the destruction and death of nerve cells that leads to the symptoms of Alzheimer’s disease.
The History Behind The Amyloid Hypothesis and Alzheimer’s
In the year 1984, George Glenner and Caine Wong, two pathologists working for the University of California, San Diego, isolated amyloid-β and determined its origin to be the amyloid precursor protein (APP). Glenner and Chang believed that amyloid-β was the cause of Alzheimer’s disease, but this hypothesis, known as the amyloid hypothesis, did not gain recognition until the discovery of inherited mutations that cause the familial form. It was not until 1991, when John Hardy, a neurogeneticist at the University College London, discovered that mutations in the gene coding for APP caused an inherited form of Alzheimer’s. Further research also revealed that the process behind the production of amyloid-β is controlled by two enzymes: β-secretase and γ-secretase. The two work together to cut the APP in two distinct places, creating a peptide fragment. In 1996, there were two more gene mutations identified, which affected the genes encoding the γ-secretase of proteins presenilin 1 and presenilin 2. These proteins influence the location at which the γ-secretase cuts the APP. The mutations in these genes result in the production of longer amyloid-β variants, which cause protein clumping and the formation of structures known as oligomers. The further accumulation of amyloid-β produces insoluble fibrils, which aggregate into the plaque characteristic of Alzheimer’s disease.

Based on the aforementioned research, there is strong evidence suggesting that amyloid-β is the cause of familial Alzheimer’s disease. The discovery of mutations proven to increase amyloid-β aggregation, along with the fact that plaques are composed of amyloid-β indicates that this protein may be involved in disease development. The mentioned aggregation of amyloid-β protein is believed to trigger processes associated with Alzheimer’s; including inflammation, tau-tangle formation, synapse dysfunction, and even cell death. Nonetheless, the most important aspect of this theory is that the familial form of Alzheimer’s is nearly identical to the sporadic form, with the key difference being that it is not as widespread and most often occurs earlier in life. Thus, the theory of amyloid-β initiating the process of Alzheimer’s development may be applied to all types of this disease.
The Main Question At Hand is the Etiopathogenesis of the Two Hallmark Pathologies Discussed in this Chapter and the Potential Causative Role of Microbes …
Alzheimer's disease in someone under the age of 65
Alzheimer's disease in someone aged 65 or older
Alzheimer's disease that is linked to someone's genes (affected families usually have members of two generations that had the disease)
A protein associated with microtubule maintenance/stability and key component of neurofibrillary tangles (insoluble fibers within brain cells)
Protein with a β-sheet secondary structure, that forms abnormal deposits in organs and tissues
The main neurotransmitter found within the body
A molecule made up of similar or identical repeating monomer (smaller molecule) copies
