9 2.2 Overview of Acute Respiratory Distress Syndrome (ARDS)
2.2.1 Epidemiology
The mortality rate of ARDS is about 40% in the patient population (Diamond et al.). The mortality rate has been slowly decreasing over the past decade, but still remains one of the highest rates among diseases (Diamond et al.). Previous studies found that about 25% of those who were ventilated were diagnosed with ARDS (Matthay et al. 2). Further research is required to decrease the number of deaths from this disease, as successful therapies have not yet been developed.
2.2.2 Causes
There are several ways in which a patient may develop ARDS. The different causes are categorized into two groups, direct or indirect (Perl et al. 2). A condition stemming from the lungs is associated to the direct cause of ARDS (Perl et al. 2). Often either viral or bacterial pneumonia is the direct cause (Perl et al. 2). Other direct causes include physical trauma to the lungs or fluid from the digestive system entering the lung space (Perl et al. 2). Conditions affecting other organs in the body may also lead to the indirect cause and development of ARDS (Perl et al. 2). The most common indirect cause is sepsis originating from other parts of the body (Matthay et al. 1). Other indirect causes include blood transfusion, overdosing on various drugs or a hemorrhage (Matthay et al. 1).
2.2.3 Diagnostic Testing
ARDS is a complex disease making it difficult to diagnose in certain cases. It requires several tests and rigorous examination to diagnose a patient with ARDS (Matthay et al. 11). ARDS patients present key features. These include increased fluid in the lungs and dangerously low amounts of oxygen in the body (Williams et al. 66). Various imaging techniques, lab tests, examination of the patient’s history, as well as physical condition are taken in order to correctly diagnose a patient (Matthay et al. 12). Common imaging techniques used are chest radiographs, computed tomography (CT) scans and lung ultrasonography (Zompatori et al. 522). Chest radiographs are most common as they are able to take the image at the bedside of ventilated patients, which provides information of the lung’s condition (Zompatori et al. 521). However, the disadvantages lie in interpreting the images, as set definitions are not given and it provides limited information about the lungs (Zompatori et al. 521). CT scans and lung ultrasonography provide much more detailed information about the patient’s lungs, but it is difficult to administer this technique, as the patient would need to be moved to the radiologist’s clinic in order to have the image processed (Zompatori et al. 522). In addition to imaging, the presence of a virus or bacteria are searched for, as in most cases this is the reason in which a patient develops ARDS (Matthay et al. 12). To identify any infection, blood cultures, lab tests and bronchoalveolar lavage fluid are assessed (Matthay et al. 12).
2.2.4 Pathophysiology
Normal and functioning lungs require several key components. These components work together for the lungs to successfully exchange carbon dioxide and oxygen to be transported either into and out of the body (Matthay et al. 3). The structure of the endothelial cells in the alveoli are important in facilitating this exchange. The epithelium cells are made up of two types of cells, which includes the type I pneumocyte and type II pneumocyte (Matthay et al. 3). Type I pneumocyte facilitates the flow and transportation of these gases into and out of the lungs, while preventing large solutes to enter the airspace (Matthay et al. 3). In congregation with type I pneumocyte, type II pneumocyte releases a substance to prevent the collapse of the alveoli (Matthay et al. 3). In addition to these cell types, alveolar macrophages and other immune cells are present to protect against foreign bodies and maintain normal function (Matthay et al. 4).
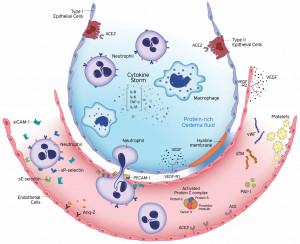
In patients with ARDS, the key feature that is observed is the loss of the epithelium and tight junction barrier (Matthay et al. 4). This causes an increase of immune cells and fluid to enter the lungs (Matthay et al. 4). The patients go on to develop oedema (Matthay et al. 4). In addition to the rise in permeability, there is an increase number of chemokines (William et al. 1). In the event of inflammation, chemokines attract immune cells to the site (William et al. 1). Due to the inflammation seen in ARDS patients, there is an increase of neutrophils (William et al. 1). Initially, it was thought that chemokine ligand 8 was solely responsible for this, but recent studies have pointed out that other chemokines may be involved (William et al. 1). Due to the increased number of cells, it becomes unmanageable for the environment (William et al. 1). Ultimately causing respiratory failure, in which patients are unable to exhale carbon dioxide resulting in the build up of it in the blood (Matthay et al. 4). In most cases, this leads to mortality (Matthay et al. 4).
2.2.5 Significance of Acute Respiratory Distress Syndrome Today

Acute respiratory distress syndrome has one of the highest mortality rates in the patient population. Today, the number of individuals developing ARDS has significantly increased due to the emergence of COVID-19 (Vassiliou et al. 14). A new study found that 85% of those who enter the ICU due to this virus go on to develop ARDS (Acosta & Singer 10). These patients require ventilation and life support as they deteriorate quickly (Vassiliou et al. 14). Recent studies are reporting that patients who require ventilation have only a 40% chance of surviving (Giamarellos-Bourboulis et al. 992). In most cases, researchers find that patients experience a ‘cytokine storm’ in the lung space (Vassiliou et al. 14). The cytokines promote an immune response and overwhelm the alveolar space causing further lung damage (Vassiliou et al. 14). Studies have identified two mechanisms that are most likely contributing to a patient’s condition (Vassiliou et al. 14). An immune dysregulation is one of the mechanisms at play (Giamarellos-Bourboulis et al. 997). The key features include a decrease in lymphocyte production, as well as an increase in cytokines (Giamarellos-Bourboulis et al. 997). In addition, IL-6 was found to be associated in the observed decrease in CD14 monocytes in the body (Giamarellos-Bourboulis et al. 997). These processes have contributed to patients developing ARDS after acquiring COVID-19.
Concept Check
This video provides a quick overview of what was discussed in this section of the chapter.
Summary
Today, acute respiratory distress syndrome is impacting many lives. It is still not a well understood disease and it is complex in its nature due to the various mechanisms involved. Because of this, effective therapies have not yet been developed, so we require a better understanding of these mechanisms to help this patient population. There are different ways in which a patient may develop ARDS, but the most common form is through a viral or bacterial infection in the lungs. ARDS is characterized as hyperinflammation in the surface of the alveoli, causing an increase of fluids and different cells such as neutrophils to enter. This ultimately harms the patient and leads to mortality.
References
“ARDS Definition (Acute Respiratory Distress Syndrome).” YouTube, YouTube, 2 Oct. 2020, www.youtube.com/watch?v=K3GI8XHThaw.
Acosta, Manuel A. Torres, and Benjamin D. Singer. “Pathogenesis of COVID-19-Induced ARDS: Implications for an Ageing Population.” European Respiratory Journal, vol. 56, no. 3, 2020, p. 2002049., doi:10.1183/13993003.02049-2020.
Adapted from “Lungs (healthy vs. diseased)”, by BioRender.com (2020). Retrieved from https://app.biorender.com/biorender-templates
Chang, Hsu-Liang, et al. “Acute Respiratory Distress Syndrome and Acute Myocarditis Developed in a Previously Healthy Adult with Influenza B.” BMC Pulmonary Medicine, vol. 16, no. 1, 2016, doi:10.1186/s12890-015-0163-3.
Diamond, Matthew, et al. “Acute Respiratory Distress Syndrome.” StatPearls, StatPearls Publishing, 18 November 2020.
Giamarellos-Bourboulis, Evangelos J., et al. “Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure.” Cell Host & Microbe, vol. 27, no. 6, 2020, doi:10.1016/j.chom.2020.04.009.
Matthay, Michael A., et al. “Acute Respiratory Distress Syndrome.” Nature Reviews Disease Primers, vol. 5, no. 1, 2019, doi:10.1038/s41572-019-0069-0.
Perl, Mario, et al. “Pathogenesis of Indirect (Secondary) Acute Lung Injury.” Expert Review of Respiratory Medicine, vol. 5, no. 1, 2011, pp. 115–126., doi:10.1586/ers.10.92.
Vassiliou, Alice G., et al. “Endothelial Damage in Acute Respiratory Distress Syndrome.” International Journal of Molecular Sciences, vol. 21, no. 22, 2020, p. 8793., doi:10.3390/ijms21228793.
Williams, Andrew E, et al. “Evidence for Chemokine Synergy during Neutrophil Migration in ARDS.” Thorax, vol. 72, no. 1, 2016, pp. 66–73., doi:10.1136/thoraxjnl-2016-208597.
Zompatori, Maurizio, et al. “Overview of Current Lung Imaging in Acute Respiratory Distress Syndrome.” European Respiratory Review, vol. 23, no. 134, 2014, pp. 519–530., doi:10.1183/09059180.00001314.