10 2.3 The Limitations of Existing Therapies and Recent Advances in ARDS Research
Learning Objectives
2.3.1 Existing therapies for ARDS
The existing therapeutic strategies for ARDS have been classified as either supportive or specific (Shaw & O’Kane 8). These supportive therapies are mainly non-pharmacological and consist of clinical management strategies that are typically useful to all critical care patients. Supportive care emphasizes the following four outcomes: (1) decreasing shunt fraction, (2) increasing oxygen delivery, (3) reducing oxygen consumption, and (4) preventing further injury (Diamond et al. 8). To do this, patients are mechanically ventilated and given diuretics to prevent fluid overload, as well as nutritional support until there are noticeable improvements. It is important to note the manner in which a patient is ventilated as some strategies may aggravate the alveolar damage and lung injury in ARDS (Diamond et al. 9). On the other hand, specific therapies focus on improving the pathophysiology of ARDS. Currently, there are no specific pharmacological therapies for ARDS and many potential interventions that showed promise in pre-clinical studies have failed to translate from bench to bedside (Shaw & O’Kane 8).
Overall, these existing interventions have improved outcomes for patients with ARDS, however, the burden of lung injury remains significant with a high incidence and risk of both morbidity and mortality. In Section 2.3.2 and 2.3.3, we will review some limitations in the understanding and management of ARDS, and then go on to discuss a few of the goals of future ARDS research.
2.3.2 Limitations in understanding the mechanisms of ARDS
ARDS is a complex syndrome that is based on a diverse etiology and various risk factors. Research has shed light on heterogeneity in the mechanism of ARDS between patients, which may be potentially contributing to the failure of prospective interventions in clinical trials (Lawler & Fan 1). As such, research has taken a more personalized approach through the stratification of ARDS patients into two distinct phenotypes, with the hopes of being able to inform clinical management strategies for these patients (Derwall et al. 1026). This notion to consider both genotype and phenotype has shown promise in the field of oncology. Particularly, a study by Ernani et al. (2017) showed great improvements in patient survival through personalized treatment of non–small-cell lung cancer with tailored immunotherapy.
Whether we can identify these ARDS subgroups at the bedside is still under investigation, however, two important concepts have come up as a result of this idea. The first is regarding ARDS phenotype subsets or subphenotypes (Shankar-Hari & McAuley 280). Subphenotypes are patient groups within a broad, heterogeneous ARDS cohort that have a similar set of observable characteristics (e.g. clinical, radiological, biological, and/or outcome characteristics). The second is of ARDS endo-phenotypes (endotypes) which are patient subsets of ARDS characterized by their underlying mechanism and/or by varying responses in treatment. Taken together, subphenotypes and endotypes could potentially help identify patient cohorts who are more likely to respond to a specific therapy (called predictive enrichment). This strategy could improve the results of future clinical trials and could help create treatments that account for individual patient heterogeneity.
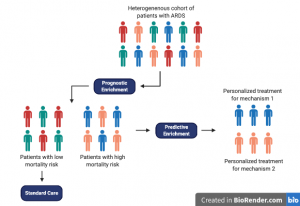
2.3.3 Goals of future ARDS research: A more personalized approach
To enable further advances in therapeutics for ARDS patients, research must address the current limitations present in the mechanistic understanding of ARDS. Some of the goals for future research in this field are outlined below.
Improved preclinical models
A better understanding of the current preclinical models of ARDS, and addressing its limitations (e.g. poor modelling of the complexity of ARDS), may help future potential interventions from failing to translate to the bedside (Horie et al. 2278). Improved experimental models would allow ineffective clinical strategies to be rejected so emphasis could be put on more promising ones.
Targeting subphenotypes of ARDS patients
Identifying patients more likely to respond to a specific treatment (i.e., predictive enrichment) should increase chances of success in clinical trials. Although randomization in experimental studies is important, this method would reduce study noise and prevent harm to patients who would not benefit from the treatment.
Latent class analysis is one way to identify subsets within ARDS cohorts. It is used to test the hypothesis that “two or more unobserved categories (latent classes) explain the relationships between observed variables in the cohort” (Shankar-Hari & McAuley 280). Calfee and colleagues (2014) have used LCA to distinguish two ARDS subgroups that have distinct characteristics, where one is classified by a greater incidence of shock, more inflammation and endothelial injury, and increased mortality. Future studies should aim to validate phenotyping methods and then work towards testing therapies in subphenotypes that are identified.
Summary
Although recent advances have significantly improved our understanding of ARDS, specific pharmacological interventions fail to demonstrate an effect in clinical trials. This failure of translation is in part due to the heterogeneity of ARDS patients, as well as the complexity of the syndrome that is not yet well represented in preclinical models. Research has been moving to a more personalized approach of ARDS where subphenotypes and endotypes are being identified to stratify ARDS cohorts. This novel approach can be used by physicians to determine in advance whether an expensive and/or harmful treatment would be effective in a specific subset of patients with ARDS.
