15 4.2. The Gut Microbiota
The Gut Microbiota
Learning Objectives
- Understanding various roles and functions of the gut microbiota
- Understand the process of microbial colonization
- Explore various diseases causes by alterations in the gut microbiome
- Understand the difference between commensal and pathogenic bacteria
- Looking at specific genus within Actinobacteria and Bacteroidetes phyla
- Understand the link between dysbiosis and disease
- Understand the effects of antibiotics on the gut microbiome
- Antibiotic resistance
- Understand the interaction between the microbiota and the host immune system
What is the Gut Microbiota?
Humans are the host to intricate communities of microbes, composed of bacteria, viruses, and eukaryotes. The gut microbiota can be found in the gastrointestinal tract, with most of its colonization in the distal gut. The gut microbiota is an important feature to human health, and the various microbes play a role in various functions including vitamin biosynthesis, energy extraction, protection against pathogen overgrowth as well as education of the immune system.

Initial microbial colonization of the gut microbiota occurs during birth and is very dynamic throughout infancy. Around the age of three, the microbiota resembles the structure that will be maintained through adulthood. Initially, following birth, the gut comprises oxygen. For this reason, the initial colonizers of the gut are aerotolerant. As the infant ages, the colonizers are replaced by anaerobes that are typical of adult gut microbiota. Major life events such as the introduction of solid foods to an infant’s diet are responsible for a large taxonomic shift in the composition of the gut. During adult life, composition remains relatively stable within an individual. There are, however, interpersonal variations between individuals. There are four primary phyla of bacteria that compose the gut microbiota. These include Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria. The gut microbiota is quantified by diversity, which is the number and abundance distributions of distinct types of organisms.
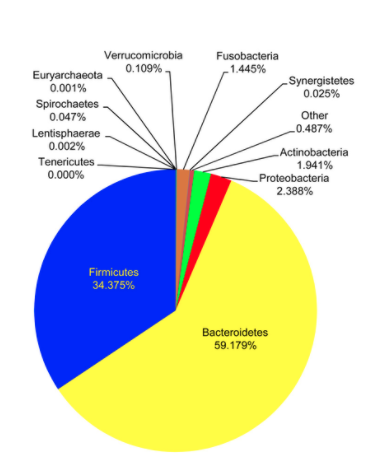
Alterations in the gut microbiota have been associated with the development of a variety of gastrointestinal and metabolic diseases; these include inflammatory bowel disease, obesity, type two diabetes, and insulin resistance. Additionally, there has been a connection identified to the Central Nervous System. This is known as the Gut-Brain-Axis in which alterations in the gut microbiome have been associated with neurological conditions such as Parkinson’s disease, autism spectrum disorder, multiple sclerosis, and now, Alzheimer’s disease.
The Ongoing War Between Commensal and Pathogenic Bacteria
Commensal bacteria compose the majority of our gut microbiota. They interact with the host and carry out functions that are important to human health. Occasionally, commensal bacteria growth rate will increase to a point that they outcompete other members of the intestinal microbiota. These are referred to as pathogenic bacteria. Another indicator of pathogenic bacteria are virulence factors that contribute to causing disease. There are a few well-known pathogenic bacteria that are capable of causing disease; these are salmonella, listeria, and E. coli.
As mentioned above, there are four main phyla that compose the gut microbiota. We will be exploring the effects of a couple of these, Bacteroidetes and Actinobacteria in particular.
Bacteroidetes consists of a large and diverse group of gram-negative bacteria in the gut. Specifically, the genus Bacteroides has been identified as increased in abundance in individuals with type II diabetes and Parkinson’s disease. This is due to the presence of lipopolysaccharide (LPS) as a major component of the outer membrane in gram-negative bacteria. LPS is capable of triggering systemic inflammation as well as the release of pro-inflammatory cytokines. As we age, our intestinal permeability increases, increasing LPS-binding proteins and, as a result, inflammation in elderly individuals, causing many issues. Some studies indicate that this inflammation contributes to Alzheimer’s disease pathology.
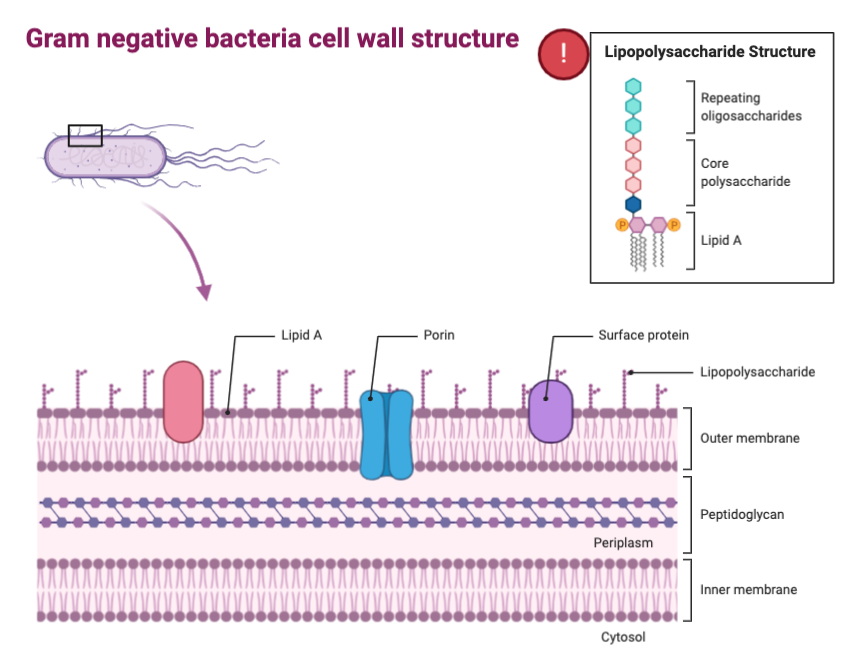
On the contrary, the phylum Actinobacteria, specifically looking at the genus Bifidobacterium, tends to work in an anti-inflammatory manner. These bacteria also tend to decrease intestinal permeability, having a protective effect against LPS-binding proteins and inflammation as a result. In a study done with mice, when supplemented with Bifidobacterium, LPS levels decreased, and gut mucosal barrier properties were improved.
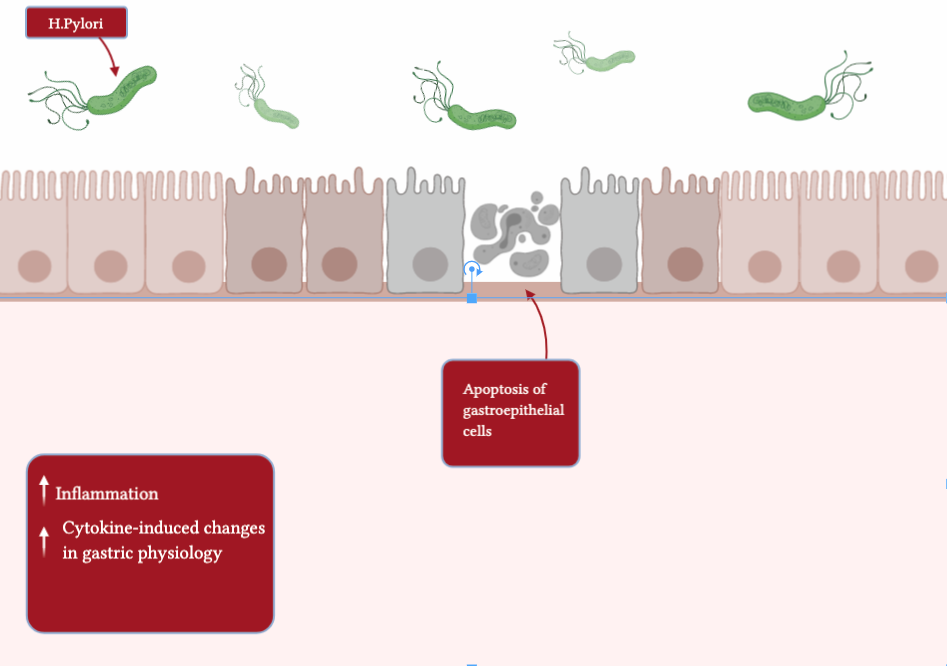
Additionally, a specific species of bacteria is known for its devastating effects on the gut microbiota. This gram-negative bacterium is known as Helicobacter Pylori (H. Pylori) and has been associated with gastric ulcers and stomach cancer. Prior to the use of antibiotics, this was a very common colonization and was found in high prevalence in developing countries. However, due to the use of antibiotics, there has been a decreased incidence of this bacterium to cause disease.
Dysbiosis and Disease
Dysbiosis refers to imbalances in the gut microbiota, these imbalances have been discovered to play a critical role in the sustainment and progression of various diseases. The mechanisms by which dysbiosis causes disease is believed to be through immune activation and systemic inflammation. There are quite a few diseases that have been associated with dysbiosis of the gut. These include autoimmune and allergic diseases, obesity, inflammatory bowel disease, and diabetes.
Specifically looking at obesity and autoimmune disorders, we can use dysbiosis to understand the cause of these diseases.
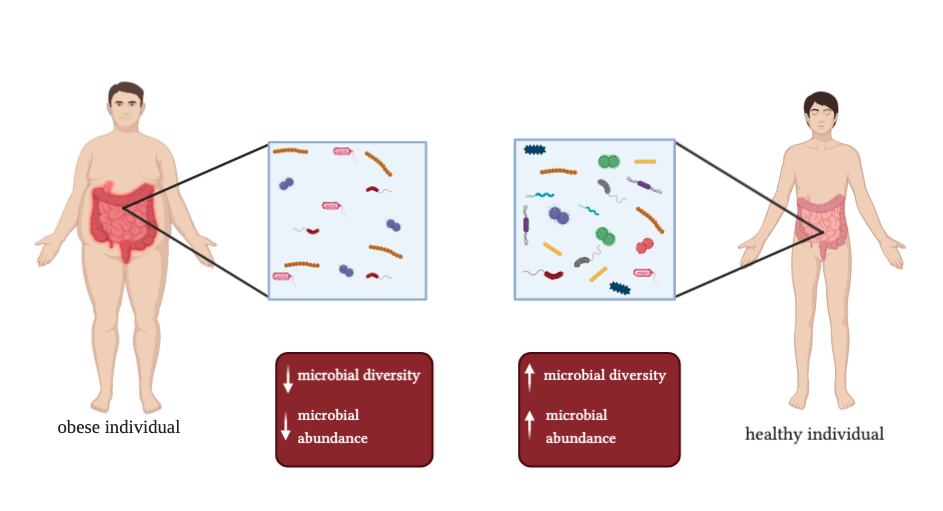
Obesity is currently a worldwide epidemic and can have many health complications. In patients with obesity, dysbiosis comes from a shift in two particular phyla, a significant reduction in Bacteroidetes and a significant increase in Firmicutes. This shift results in low-level inflammation. This low-level inflammation is chronic and is hypothesized to be the cause of metabolic syndrome and obesity.
Autoimmune diseases are also thought to be associated with the gut microbiota as the microbiota plays a role in developing normal immune system functions. Looking specifically at type 1 diabetes, similarly to obesity, there have been phyla identified that shift to cause dysbiosis. Children with a high genetic risk of type 1 diabetes have been found to have an increase in Bacteroides, a genus within Bacteroidetes, and a strain of Firmicutes known as CO19. Additionally, these children are also found to have relatively decreased diversity. The role of gut microbiota dysbiosis and autoimmune diseases can be confirmed with the use of germ-free mice. After experimentation, it can be found that autoimmune diseases do not develop in germ-free mice. However, the disease can be restored when microbial colonization of these germ-free mice occurs. In particular, these diseases have been thought to be mediated by the incorrect actions of the adaptive immune system, which have been educated through the gut microbiota.
The Effects of Antibiotic on Gut Microbiota
It is well known that antibiotics have a strong effect on the gut microbiota. Recently, there has been talk about their misuse and overuse in the development of antibiotic-resistant bacteria. Following the use of antibiotics, there are often major modifications in the composition of the gut microbiome. Bacterial diversity is reduced, and it can take months for some of the taxa to recolonize. Often, as recolonization of the gut occurs post-antibiotic use, the gut is even more susceptible to dysbiosis and disease. This is due to the increased susceptibility of the gut to allow various microbes to over-grow the commensal bacteria, as well as the overall reduced resistance of the gut to being colonized. These modifications from antibiotic use can cause long-lasting changes in the composition of the gut.
The Interaction Between Gut Microbiota and Host Immune System
The development of the immune system depends on the gut microbiome for education. Our immune system can be further divided into the innate immune system and the adaptive immune system.
During development, both the innate and adaptive immune systems require interactions with the gut microbiome. The innate immune system is regulated by the gut microbiota in order to ensure the microbiota is maintained, ultimately advancing their fitness within the gastrointestinal tract. The adaptive immune system is also arranged by the gut microbial community. T-cells are one of the major components of the adaptive immune system and these cells require education and interactions from various microbes in order to function normally. Additionally, the commensal bacteria within the gut have a role in suppressing the inflammatory response as well as with the promotion of immunological tolerance. The Hygiene hypothesis is also another indication that gut microbial colonization is required for the immune system to develop and function properly. The hypothesis states:
“the lack of exposure to pathogenic and non-pathogenic microbes early in life might result in an asthmatic phenotype due to impaired development of the immune system.”
Living in close association such that one species benefits without harming the other.
