62 6.4 Limitations and Future Directions for CAR T Cells
Limitations of CAR T Cells
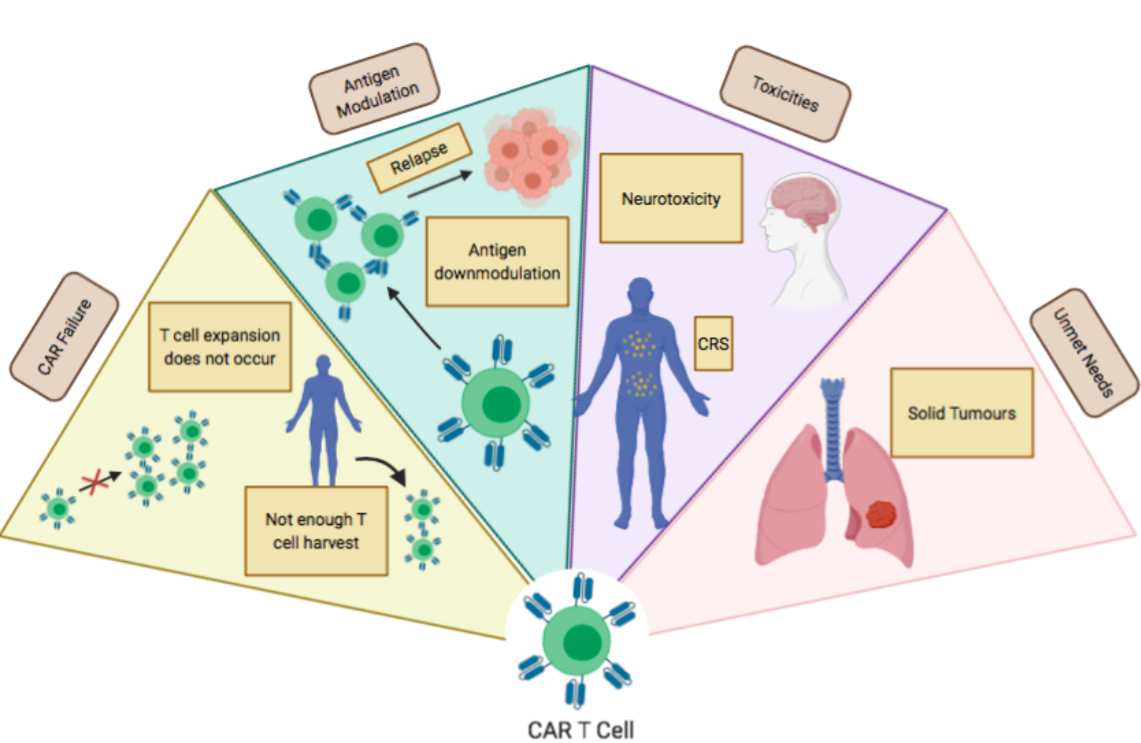
In the previous section, Liu and colleagues’ paper demonstrated that CAR T-cells could benefit both patients with PD-L1high tumours and those with PD-L1low tumours (Liu et al.). However, CAR T-cell therapy may not always be effective and can fail due to a variety of reasons. For example, it may not always be possible to harvest enough T cells. Another limitation is that CAR T cells may not expand properly. Additionally, antigen downmodulation can also occur, resulting in resistance to CAR T cell therapy. CAR T cell therapy can also cause toxic side effects, including cytokine-release syndrome and neurotoxicity, which can be fatal in some cases. Finally, in past studies, CAR T cells were not very effective against solid tumours such as NSCLC (Shah and Fry).
Future Directions
Working With Immunocompetent Models
Since Liu et al.’s study was only performed on immunodeficient mice, it is uncertain whether anti-tumour effects will persist in the presence of the immune system. The next step for this study would be to test the effects of PD-L1 CAR T cell therapy in immunocompetent, syngeneic tumor models, such as C57BL/6 mice. This will allow researchers to test the effectiveness of PD-L1 CAR T cell therapy treatment at different stages of tumour development and in the presence of intact innate and adaptive immune systems (Chulpanova et al.). Though adverse events were not seen in immunodeficient mice, further research on adverse events and off-target toxicity, such as neurotoxicity, should be conducted in immunocompetent models to assess the safety of CAR T cell therapy on non-tumour cells.
Targeting the tumour microenvironment
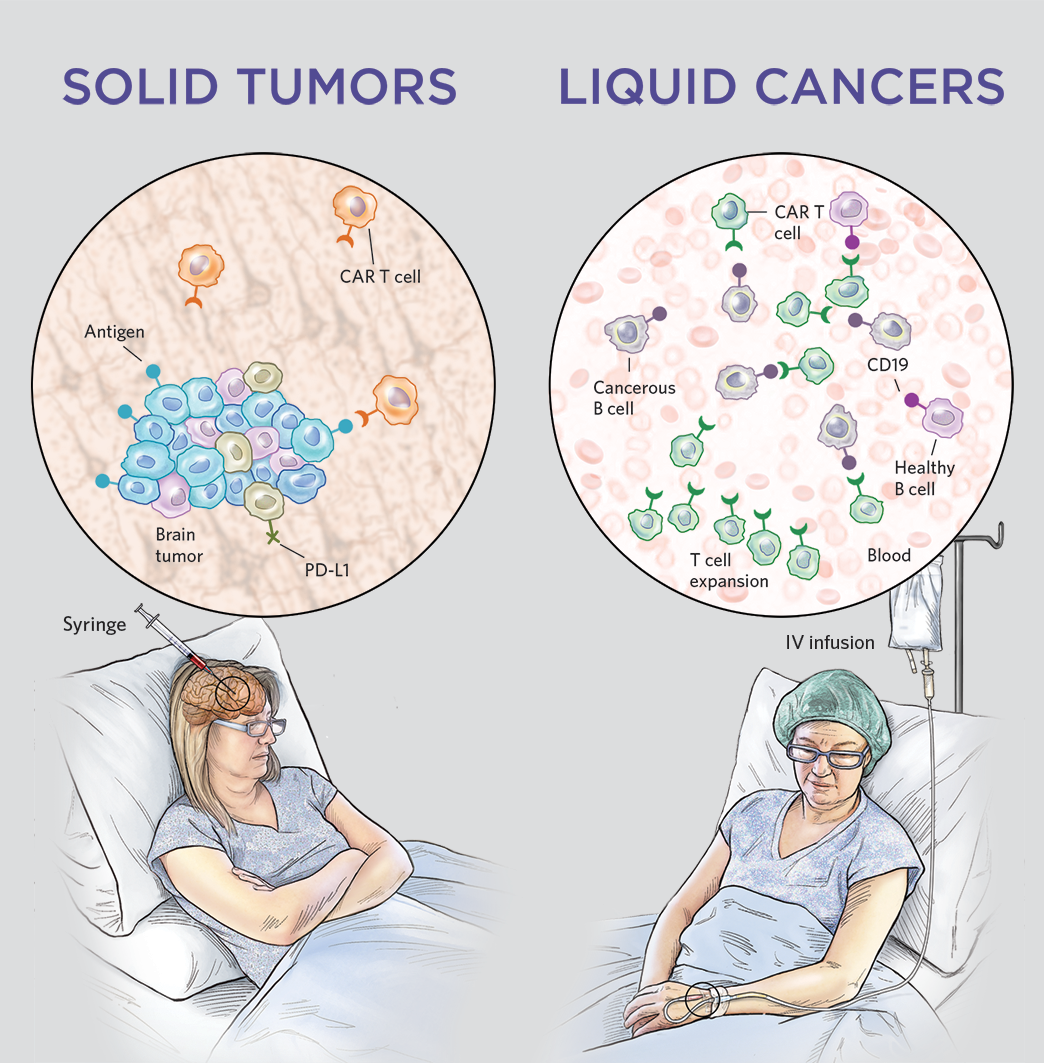
A major caveat with using CAR T cell therapy on solid tumours is the lack of targetable and reliable tumor-specific antigens. Additionally, rapid tumour growth and varying expression levels of surface antigens on tumour cells make it challenging to target with CAR T cell therapy (Grens). An alternative to finding tumour-specific antigens is engineering CAR T cells to target the tumour microenvironment. In a study by Xie and colleagues, they created nanobody-based CAR T cells to target markers in the tumour microenvironment, such as PD-L1 and EIIIB, rather than the tumour directly (Xie et al.). Through interfering with the tumour extracellular matrix and vasculature, which supplies nutrients for tumour growth, PD-L1 CAR T cells and EIIIB CAR T cells were able to limit tumour growth and improve the survival of immunocompetent mice (Xie et al.). In combination with other therapies, this proposed mechanism is promising as CAR T cells would be responsible for controlling tumor size, while the other therapy eradicates the tumour. Furthermore, using CAR T cells that target the tumour microenvironment may be a potential treatment for advanced stages of NSCLC involving metastases, since PD-L1 and EIIIB are commonly found in multiple solid tumour types (Xie et al.).
References
Chulpanova, Daria S., et al. “Mouse Tumor Models for Advanced Cancer Immunotherapy.” International Journal of Molecular Sciences, vol. 21, no. 11, June 2020. PubMed Central, doi:10.3390/ijms21114118.
Grens, Kerry. “The Next Frontier of CAR T-Cell Therapy: Solid Tumors.” The Scientist Magazine®, https://www.the-scientist.com/features/the-next-frontier-of-car-t-cell-therapy–solid-tumors-65612. Accessed 3 Dec. 2020.
Liu, Ming, et al. “Targeting PD-L1 in Non-Small Cell Lung Cancer Using CAR T Cells.” Oncogenesis, vol. 9, no. 8, 8, Nature Publishing Group, Aug. 2020, pp. 1–11. www.nature.com, doi:10.1038/s41389-020-00257-z.
O’Keefe, Laurie. Solid Versus Liquid Cancers. https://www.the-scientist.com/features/the-next-frontier-of-car-t-cell-therapy–solid-tumors-65612. Accessed 3 Dec. 2020.
Shah, Nirali N., and Terry J. Fry. “Mechanisms of Resistance to CAR T Cell Therapy.” Nature Reviews Clinical Oncology, vol. 16, 5 Mar. 2019, pp. 372–385., doi:10.1038/s41571-019-0184-6.
Xie, Yushu Joy, et al. “Nanobody-Based CAR T Cells That Target the Tumor Microenvironment Inhibit the Growth of Solid Tumors in Immunocompetent Mice.” Proceedings of the National Academy of Sciences of the United States of America, vol. 116, no. 16, 16 2019, pp. 7624–31. PubMed, doi:10.1073/pnas.1817147116.
