28 3.7 Honeybee Venom and Melittin Suppress RTK Phosphorylation
Overview
Many TNBC and HER-2 enriched cells express epidermal factor receptor (EGFR) and human epidermal growth factor-2 (HER-2), respectively (Duffy et al., 2020). These receptors are part of the receptor tyrosine kinase (RTK) family which have various intracellular signalling modalities that confer oncogenic potential to human breast cancer cells. Here, we will seek to examine the role of both honeybee venom and melittin on the disruption of these pathways.
Watch this video to learn more about the P13K and MAPK downstream signalling pathway in HER2 Breast Cancer:
Downregulation of RTK Phosphorylation: Immunoblot Assay
Authors treated SKBR3 cell lines expressing EGFR+ and HER2+ and SUM159 cell lines expressing EGFR+ with IC50 of honeybee venom or melittin. They looked at mRNA expression levels of various phosphorylation sites of RTKs and common oncogenic signalling pathways using Western Blot.
In the SKBR3+ cells (left fig. below), both compounds strongly downregulated the phosphorylation of RTKs (EGFR+ and HER+) as well as serine/threonine-specific protein kinases, Akt and mitogen-activated protein kinase (MAPK) signalling pathways (Duffy et al., 2020).
Similarly, in the SUM159 extracts (right fig. below), both compounds strongly downregulated the phosphorylation of EGFR+; however, only melittin peptide downregulated the Akt pathway. In addition, some pathways have been upregulated, such as the MAPK. Authors suggest this may have been due to a shift towards a negative feedback state that causes signalling molecules such as Erk1/2 to be activated as a means to protect the cell from apoptosis (Duffy et al., 2020).

Observing Substrate Specificity of Melittin to EGFR: BRET Assay
EGFR signalling is one of the main players involved in breast cancer therefore the authors wanted to investigate whether melittin interfered with the EGFR binding site by observing the substrate saturation using the BRET assay (Duffy et al., 2020). The authors took HEK293 cell lines and transfected them with a fluorescent plasmid containing cDNA for EGFR. This allowed them to visualize the interaction in an efficient manner.
Concept and Methodology Review
Theory: Substrate Saturation Curve
A saturation curve enables one to detect whether the presence of a particular substrate reaches a plateau. This indicates when there are no available active binding sites thereby causing the surrounding environment to become “saturated”; a typical representation of normal enzymatic activity.
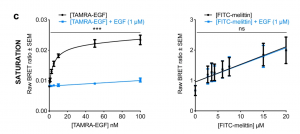
Melittin Does Not Interfere with the Binding of EGF to their Cognate Receptor
When authors added 1 uM of additional EGF in the presence of melittin, there was no indication of saturation represented by the linear graph above. Furthermore, no difference was observed between with or without the addition of EGF. Hence, the authors concluded melittin does not bind to the growth factor binding sites. This supported their notion that melittin modulates downstream signalling pathways in HER-2 and EGFR-enriched breast cancer cells by incorporating into the cancer cell plasma membrane via their hydrophobic C-terminus and remote from the receptor tyrosine kinases (Duffy et al., 2020).

Key Takeaways
- Honeybee venom and melittin have shown to substantially downregulate the phosphorylation of RTKs and their associated oncogenic signalling pathways
- However, MAPK was upregulated by both honeybee venom and melittin in SUM159 cells. This suggests some pathways have other collateral effects aside from their involvement in cell activation and/or proliferation.
- Molecular interactions between two proteins can be monitored using Bioluminescence resonance energy transfer (BRET).
- Melittin does not interfere with EGF – does not act as a competitor substrate at EGFR binding sites.
- Melittin can integrate into the plasma membrane of breast cancer cells via their hydrophobic C-terminus thereby reducing dimerization of receptor tyrosine kinases.
- This correspondingly downregulates the downstream pathway involving P13K/Akt.
References
- Thomas, P., & Smart, T. G. (2005). HEK293 cell line: a vehicle for the expression of recombinant proteins. Journal of pharmacological and toxicological methods, 51(3), 187–200. https://doi.org/10.1016/j.vascn.2004.08.014
