Fuel Oil Supply Systems
Formation of Petroleum and Natural Gas
Formation of Petroleum and Natural Gas is through the accumulation of organic material-which is typically marine mud, and burial and preservation of organic material made through reducing conditions.
-
- Black, organic-rich mud is buried deeper and converted to rock – shale
- After burial, the organic matter is heated
- When heat is sufficient – in the range of 100-300 oC – the organic matter is “cooked” and oil forms
- Process is called thermal maturation
If heat is greater than 300 degrees C, the liquid petroleum is further broken down to form natural gas. However, if heat is too great, even the natural gas is broken down to form carbon dioxide, which has no value as a fuel.
Migration and Concentration
-
- Petroleum must leave source rock
- Migration is essential to enable easy extraction of the oil
- To be economically concentrated, petroleum must migrate to a reservoir rock with a trap
Distribution and Refining of Petroleum
Economic accumulations of petroleum only occur when all of the conditions are met
-
- Conditions are most likely where there are thick accumulations of sedimentary rock – in sedimentary basins
Some of the world’s most productive sedimentary basins
- Saudi Arabia
- Kuwait
- Alaska – north slope
- Texas – Louisiana Gulf Coast
- Iraq and Iran
- Mexico
- Venezuela
Video: Alberta Tar Sands & Sudbury Basin
Watch the video “Alberta Tar Sands & Sudbury Basin” [ 7:51].
Link of Interest: Visit the Energy Fact Book from the Natural Resources of Canada
Heat and/or chemical treatment to produce gasoline, diesel Fuel, kerosene, liquified Propane (LPG), and petroleum bases for plastics.
|
Types of solid fuel
|
Types of liquid fuel
|
Types of gaseous fuels
|
Fuel Oils (Light and Heavy)
Fuel oils are refined either by distillation or by cracking.
Cracking is a process where heavy hydrocarbons are broken down into simpler molecules. Cracking, also referred to as pyrolysis, is the breakdown of a large alkane into smaller, more useful alkenes and an alkane.
Methods of Cracking:
-
- Fluid Catalytic Cracking
- uses a catalyst to break down molecules;
- Hydrocracking
- catalytic cracking process assisted by the presence of an elevated partial pressure of hydrogen gas;
- Steam Cracking
- petrochemical process in which saturated hydrocarbons are broken down into smaller hydrocarbons
- Fluid Catalytic Cracking
Distillation is the process used to separate oil into its constituent parts.
-
-
- Condensed liquids are termed distillates.
- The residual oils are the bottoms or crude left after distillation.
- The residuals can also be blends of residuals and distillates.
-
Fuel Oils
Lighter Oils
Heavier Oils
|
Fuel oils no. 1 and no. 2 are distillate fuels which consist of distilled process streams. Residual fuel oils no. 4 have residues remaining after distillation or cracking and are made up of lends of such residues with distillates.
All fuel oils consist of complex mixtures of hydrocarbons. They can be Aliphatic- hydrocarbons that don’t contain a “benzene ring” or Aromatic-hydrocarbons which contain one or more benzene rings.
All fuel oils consist of complex mixtures of hydrocarbons.
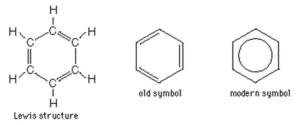
Aliphatic- Hydrocarbons that don’t contain a “benzene ring”
Aromatic- Hydrocarbons which contain one or more benzene rings.
Benzene Ring
A six-membered carbon ring with alternating double and single bonds. Named for benzene – C6H6
Benzene, C6H6, is the simplest aromatic hydrocarbon and was first discovered with this ring structure.
Aromatic Hydrocarbons
General properties:
-
-
- Display aromaticity – sweet scent.
- The carbon-hydrogen ratio is high.
- They burn with a sooty yellow flame because of the high carbon-hydrogen ratio.
-
Aliphatic Hydrocarbons
Carbon atoms joined together in straight chains, branched chains, or rings (alicyclic).
Can be joined by:
-
-
-
-
- single bonds (alkanes)
- double bonds (alkenes)
- triple bonds (alkynes).
- Other elements can be bound to the carbon chain
-
-
-
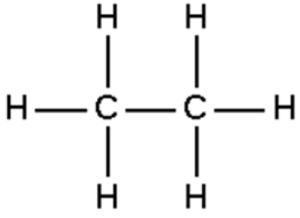
| Ethane
|
Isobutane
|
Methane
|
Alkanes
The Generic chemical formula
CnH2n+n where n = 1, 2, 3…
Example: Methane is CH4
i.e., Methane, butane, propane, ethane
They can be saturated: no more hydrogen atoms can be added to the molecule.
Fuel Oils No. 1 and 2.
Diesel fuel no. 1 and no. 2 are similar in chemical composition to fuel oil.
Recall the properties of fuel oil are: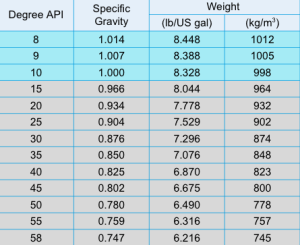
-
- Specific gravity
- Specific gravity is important because oil is sold by volume.
- Fuel typically sold with Specific gravity at 15°
- Heating value
- Viscosity
- High viscosity – high resistance to flow
- Low viscosity – low resistance to flow
- Viscosity varies inversely with temperature
- Flash point
- Fire point
- Pour point
- Specific gravity

Fuel oil no. 1 (kerosene)
- a light distillate which consists primarily of hydrocarbons in the C9 – C16 range
- Made up of C11 through C12 hydrocarbons
- Thin oil obtained from the fractional distillation of crude oil
- A mixture of hydrocarbons of the alkane (single bond) series
- Highly refined form is used in jet aircraft fuel.
Fuel oil no. 2
- a heavier, usually blended, distillate with hydrocarbons in the C11 – C20 range.
Fuel Oil Grades
The ASTM (American Society for Testing and Materials) classifies fuel oils by grade number. Grades range from Grade 1 to Grade 6. Grade 1 being the lightest and Grade 6 the most viscous (heavy).
Light fuel oil (LFO) is light in color and has an average specific gravity in the range of 0.82 to 0.86. It can be used without preheating.
Composed mostly of:
- Carbon (86% wt.)
- Hydrogen (13% wt.)
- Sulphur (0.1 to 0.2% wt.).
- Also contains trace amounts of ash and sediments.
Grade 0
|
Grade No. 1
|
Grade No. 2
|
Production, Imports And Exports Light Fuel Oil in Canada
Review the line graph from 1985 to 2001 of light fuel oil imports in Canada

Heavy fuel oil (HFO) is a mixture of hydrocarbons composed of residual fractions from crude oil distillation and processing. Heavy Oil is characterized by it’s black colour, a high specific gravity (0.92 to 0.98), and a high viscosity.
HFO is Composed mostly of:
-
- Carbon (86% wt.)
- Hydrogen (11% wt.)
- Sulphur (2% wt.).
- Contains other impurities:
- Ash, metals and water.
- HFO needs to be preheated.
Heavy Fuel Oil Grading:
Grade No. 4
|
Grade No. 5 (light)
|
Grade No. 5 (heavy)
|
Grade No. 6 is also called “bunker C”
|
Production, Imports And Exports Heavy Fuel Oil in Canada
Review the line graph from 1985 to 2001 of heavy fuel oil imports in Canada

| Fuel Type | Fuel Consumption (m3) |
Sulphur Mass (tonnes) |
Average Sulphur Content (% wt) |
Distribution of Sulphur in Fuel (%) |
| Heavy Fuel Oil | 9,157,390 | 160,565 | 1.727 | 82.0 |
| Motor Gasoline | 38,911,587 | 8,168 | 0.029 | 4.2 |
| Light fuel Oil | 4,565,310 | 10,607 | 0.201 | 5.4 |
| Diesel Fuel | 3,500,151 | 7,412 | 0.249 | 3.8 |
| Low Sulphur Diesel Fuel | 20,886,595 | 5,899 | 0.034 | 3.0 |
| Aviation Gasoline | 125,198 | 5 | 0.005 | 0.0 |
| Others | 7,392,711 | 3,038 | 0.051 |
Sulphur in Fuel in Canada
There is currently no regulated national standard for sulphur in either HFO or LFO. British Columbia, Ontario, Quebec, New Brunswick and Montreal regulate the sulphur content in HFO at levels ranging from 1.1% wt. up to 3.0% wt. While New Brunswick, Ontario and Quebec regulate the sulphur content of LFO at 0.5% wt.
Sulphur Content varies within each grade of oil.
-
-
- Lowest in Grade No. 1 (0.01 % to 0.5% by weight)
- Highest in Grade No. 6 (0.7-3.5%).
-
Sulphur content should be kept to a minimum due to corrosion and sulphur dioxide emissions.
Impurities such as water and sediment content determined by centrifuging a sample of oil. They should not be more than 2%. Incombustible impurities in oil include natural salts, chemicals used in refining operations, and rust and scale picked up in transit show up as ash.
Power Plant Systems
Fuel Oil Supply Systems
A typical fuel handling equipment is composed of:
- Strainers
- Oil heaters
- Pumps
- Automatic regulators
- Auxiliary equipment
- Instrumentation.
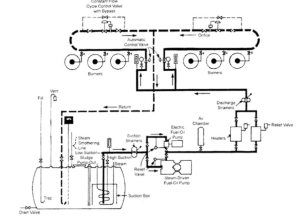
Illustration of an oil-handling system.Activity: Oil Handling System Diagram
Click on the image hotspots to explore the functions for each part of the oil-handling system.
Let’s take a look at some of the major parts of the oil-handling system including Strainers, Pumps, Heaters and Control Valves.
Fuel Oil Pumps
A pump must be able to deliver sufficient fuel oil pressure to the burners. This can be achieved 2 ways:
1. Mechanical Atomization
- Pressure required ranges from 345 to 1725 kPa.
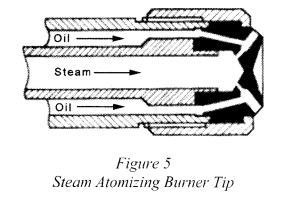
2. Steam Atomization
- Pressure required ranges from 15 to 860 kPa.
Activity: Fuel Pump Images
Click the arrow to view the fuel pumps.

Strainers
Typically there are two sets of basket type duplex strainers in a fuel oil system.
The first set is found before the fuel oil pump and has a coarser screen. The second set is found after the fuel oil heaters (discharge strainer) and typically have a fine mesh screen.
Differential pressure gauge and/or switch which reads the pressure drop across the strainers.
Fuel Oil Heaters
Steam atomization is used in oil burners to assist the mixing of oil and air to promote full combustion. With steam injection, the oil is reduced to a fine spray that mixes with air and burns easily. The result is a flame that is short and intense. The exchanger must be sized so that it can heat the oil up to 85 °C from 38 °C. In Mechanical atomization the oil needs to be heated up to 104 °C.
Automatic Control Valve
Combustion control system signals the control valves to supply the required flow of oil to the banks of burners. The oil is fed in proportion to the supply of combustion air required for stable firing.
Constant Flow Cycle Control Valve
For mechanical atomizing burners, the constant flow cycle control valve maintains a pressure sufficient for the oil to be mechanically atomized.
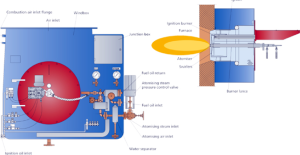
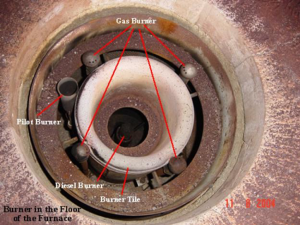
Burners
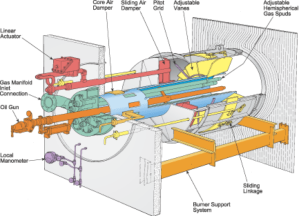
A furnace burner is a component of a furnace where air mixes with fuel, and is burned in order to create heat. This heat is then distributed throughout the home via pipes or ductwork.
The pilot burner is positioned to light either the gas inner spuds or the oil atomizer.The gas header supplies the gas inner spuds and the outer spuds, which extend beyond the burner throat. Spuds are the gas nozzles that give the flame its location and shape. A “spud” is used to meter gas at the proper flow rate at each burner.
An important component of gas furnaces is the burner – a furnace may have one, or multiple. Recall, the burner is the component where gas mixes with air then is burned to create heat.