39 FDA Crisis for Pfizer: The Impact of an FDA Warning On the Company
Hetal Patel
April 9th, 2019
On an early February morning, researchers running the post-marketing clinical trial for Pfizer’s rheumatoid arthritis drug, Xeljanz, had discovered a potential safety concern. After notifying the FDA, a safety alert had to be issued. Pfizer’s media team got to work on writing up a statement for immediate release.
About Rheumatoid Arthritis
Rheumatoid arthritis is a common chronic autoimmune disease that affects 1 in 6 Canadians over the age of 15 (UCB Canada, 2019). On a global scale, its prevalence is around 0.3-1% (World Health Organization, 2016). As the population ages, this number is expected to increase (Cross et al., 2014). Rheumatoid arthritis occurs when the immune system starts to attack its own body tissue (Mayo Clinic, 2019). Currently, the trigger for this immune response is unknown (Mayo Clinic, 2019). The disease is associated with inflammation of the synovial membrane, autoantibody production, and deterioration of bone and cartilage around the joint (Mayo Clinic, 2019). This disease generally occurs around ages 20-40 and can be a disabling condition as it can cause deformities (World Health Organization, 2016). Rheumatoid arthritis also increases the potential for developing things like cardiovascular complications, infections, and cancer (Mayo Clinic, 2019).
Currently, there is no cure for rheumatoid arthritis, however early treatment of the disease has been shown to help with the remission of the symptoms (Mayo Clinic, 2019). Typically the drugs prescribed are disease-modifying anti-rheumatic drugs (DMARDs) (Mayo Clinic, 2019). These drugs work by slowing down the progression of the disease to prevent further damage to joints and tissue (Mayo Clinic, 2019). Examples of the most commonly prescribed DMARDs include methotrexate, leflunomide, and hydroxychloroquine (Mayo Clinic, 2019). A newer class of DMARDs falls into the biologic categories. They work by targeting the part of the immune system that triggers the inflammatory response in joints (Mayo Clinic, 2019).
Xeljanz
Xeljanz (tofacitinib) is a biologic drug developed by Pfizer for the treatment of rheumatoid arthritis. This drug differs from conventional treatment methods as it is a Janus kinase inhibitor. Janus kinase (JAK) is an enzyme that is part of the pro-inflammatory response (Macfarlane & Todd, 2014). Xeljanz works by inhibiting the JAK enzyme from sending off a pro-inflammatory signal (Macfarlane & Todd, 2014). Xeljanz comes in a tablet format, therefore it offers a new route of administration as the previous therapeutics were injectables (Baldock, Baynton, Baskett, & Bailey, 2018). Xeljanz is the first JAK inhibitor to be approved by both the FDA and Health Canada for the treatment of rheumatoid arthritis (Drugs.com, 2016; Pfizer, 2018)
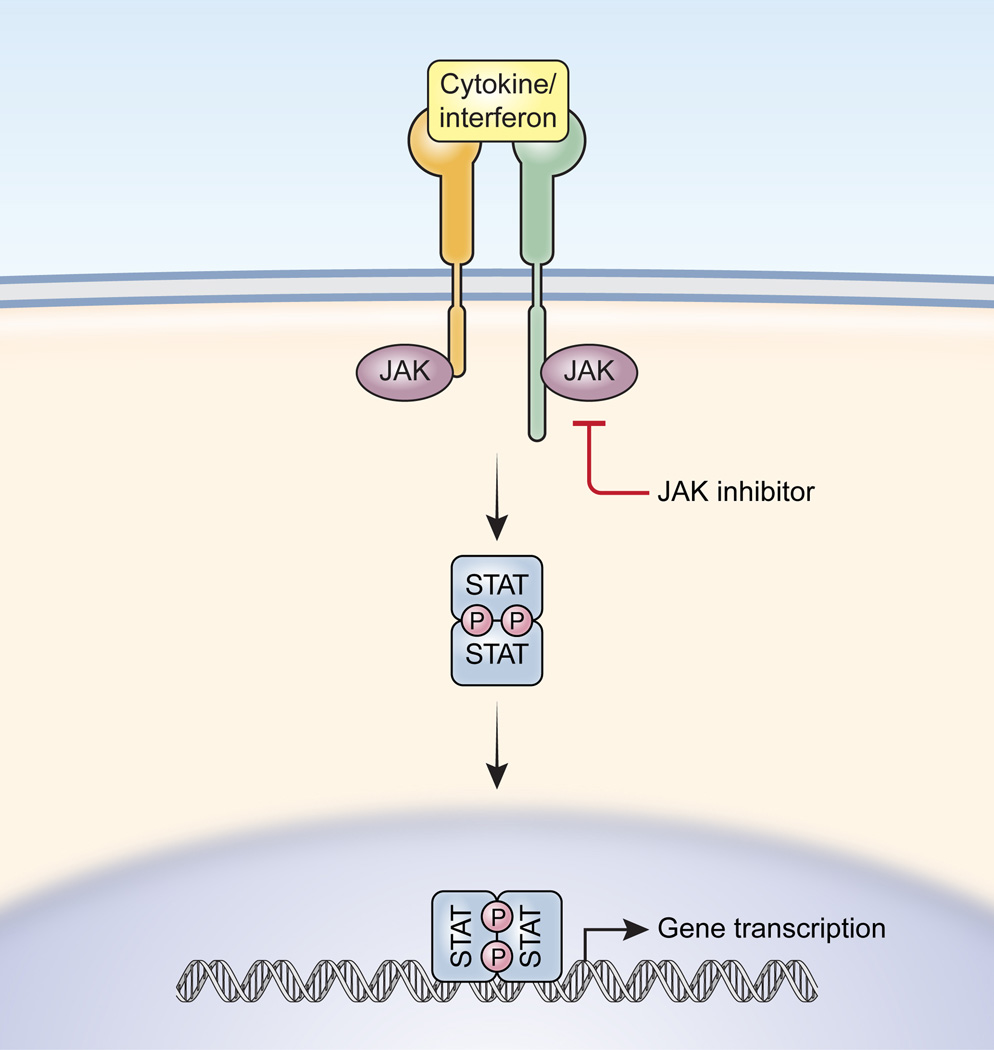
The JAK inhibitor binds to the active site, therefore preventing any downstream reactions from occuring. This ultimately prevents the production of pro-inflammatory compounds (Gadina, 2013).
FDA Approval Timeline
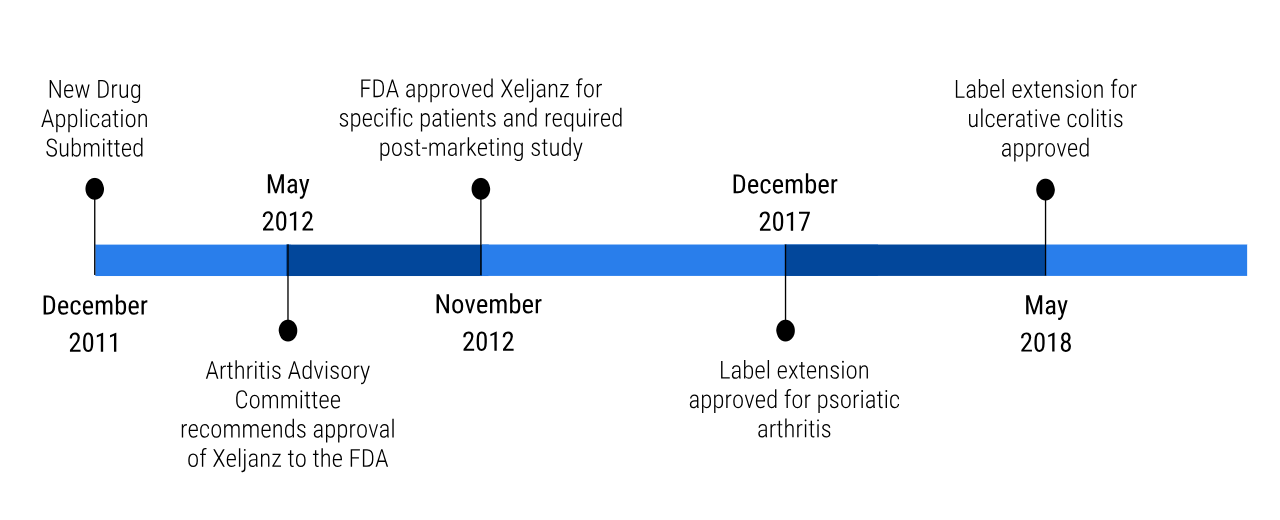
Once the phase III clinical trials were completed, Pfizer first sent in their new drug application for Xeljanz in December of 2011. Then in May 2012, the Arthritis Advisory Committee recommended the approval of Xeljanz to the FDA. Six months later, the FDA had approved the drug for patients that had Rheumatoid Arthritis and did not respond well or could not tolerate to methotrexate (Pfizer, 2012). However, the FDA did require that there is a boxed warning on the product to highlight the safety concerns with the drug (Drugs.com, 2012). Additionally, the FDA also wanted Pfizer to conduct a post-marketing clinical trial (Drugs.com, 2012). These studies are done to learn more about the long term effects and other safety concerns related to Xeljanz (Drugs.com, 2012; FDA Biologics Evaluation and Research, 2018). These safety concerns include cardiovascular events, cancer, and certain infections (Drugs.com, 2012). The study looks at patients over the age of 50 with one cardiovascular risk factor (Pfizer, 2019). Pfizer also wanted to get a label extension on Xeljanz. Label extensions essentially allow the drug to be utilized for the treatment of other diseases. The first extension that was approved by the FDA was for treatment of psoriatic arthritis in 2016 (Drugs.com, 2017). The second extension that was approved was for the treatment of ulcerative colitis in May 2018 (Drugs.com, 2018). The higher dose of 10mg twice a day is approved for ulcerative colitis patients (Baldock et al., 2018).
Performance of Xeljanz
Other than the post-marketing study, the approval was obviously great news for Pfizer. Everything at this point seems to be looking up and the drug was set to be very profitable. Researchers had said that the JAK class of drugs was expected to more effective than the other classes of drugs used in this area (Helfand, 2018). Analysts had predicted that this drug was set to disrupt the market for rheumatoid arthritis anti-inflammatories (Helfand, 2018). They had stated that the drug was going to take up 24% of the rheumatoid arthritis market (Helfand, 2018). The analysts predicted correctly as Xeljanz quickly became one of Pfizer’s leading brands in their innovative health segment. It even exceeded the profit estimates for the second quarter of 2018 (CNBC, 2018). By the end of 2018, Xeljanz sales were up 37% compared to 2017, which was primarily from rheumatoid arthritis sales (Pfizer, 2019a). Pfizer had reported that they expected strong growth into 2019 with the help of key products including Xeljanz (Pfizer, 2019a). With this, they decided to spend over 60 million on commercials in December and January (Bulik, 2019). These commercials were targeting patients with rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.
Safety Warning Issued
In February of 2018, the Rheumatology Data Safety Monitoring Board (DSMB) issued a safety alert in response to a link found between the higher dose of Xeljanz (10mg twice a day) and an increase in pulmonary embolisms (Pfizer, 2019). This prompted the FDA to issue a safety alert for the drug. In response, Pfizer reduced the dose the patients in the trial to 5mg twice a day. Following the FDA’s lead, Health Canada issued its own alert for the drug (Government of Canada, Health Canada, 2019). The European Medicines Agency was not far behind and did the same (Liu, 2019).
Implications
The warning issued by the FDA comes at a critical time for Pfizer for many reasons. The first being that Pfizer will soon lose exclusivity for one of its blockbuster drugs called Lyrica in June of 2019. This drug generated close to $5 Billion (USD) in 2018 alone (Forbes, 2019). It is expected that the losses in sales from this will impact Pfizer into 2020 (Crumly, 2019). The impact on their financials will be huge since they are expected to lose over two billion in Lyrica sales this year (Pharmaceutical Technology, 2018). This means that Pfizer needs other drugs to perform well to help meet their financial goals in the future.
Another concerning factor that puts more pressure on the performance of Xeljanz is that their current rheumatoid arthritis drug called Enbrel is experiencing a decline in sales. This decline has actually brought down the companies inflammation and immunology portfolio (Packer-Tursman, 2017). The decline comes after the availability of the first biosimilar for Enbrel and reductions in prices in Europe (Market Realist, 2016). Thus, Pfizer was expecting Xeljanz to soften the blow of the decline (Packer-Tursman, 2017).
Lastly, the fact that other pharmaceutical companies are coming out with their own JAK inhibitors also puts a great deal of pressure on the performance of Xeljanz. The first company to release a JAK inhibitor after Pfizer was Eli Lilly, with their drug Olumiant, in June 2018 (Pharmaceutical Technology, 2018a). AbbVie and Gilead/Galapagos are currently in the late stage approval process for their JAK inhibitors (Pharmaceutical Technology, 2018a). The drugs offered from AbbVie and Gilead/Galapagos are the biggest threat as they have better safety profiles when compared to Xeljanz (Pharmaceutical Technology, 2018a). More specifically, the drug being developed by Gilead/Galapagos was actually found to have a low incidence of blood clots (Taylor, 2019), which is the side effect currently plaguing Xeljanz.
Class-wide Issue or Just a Xeljanz Issue?
The increase in pulmonary embolisms may not be directly caused by Xeljanz. In fact, the root of the pulmonary embolisms might be a class-wide issue, meaning it would be associated with all JAK inhibitors (Market Insiders, 2018). This also means that other companies will be facing similar regulatory hurdles when trying to get to market. However, the fact that Xeljanz already has a black box warning on the label causes more concerns with patients and physicians. Adding on another warning about the potential for blood clots could make selling Xeljanz much harder (Renauer, 2019).
The Future
It is very clear that the FDA warning comes at a critical time for Pfizer. The post-marketing clinical trial is set to be completed later in 2019 (Liu, 2019). The conclusions from the study could potentially result in Pfizer pulling the stronger (10mg twice a day) dose from the market, discontinuing the use of Xeljanz for rheumatoid arthritis, or completely removing the drug from the market.
If the higher dose is banned, it could impact sales from ulcerative colitis. The higher dose was only just recently approved for the treatment of ulcerative colitis. Patients on this medication are required to take 10mg twice a day for the first couple of weeks for effective therapy (Renauer, 2019). Since it is new on the market for ulcerative colitis, there are concerns with the fact that this warning will impact Pfizer’s ability to grab a good portion of that market (Renauer, 2019).
The impact on Pfizer could be substantial if the results of the study conclude that the drug should no longer be used for the treatment of rheumatoid arthritis. As mentioned, the drug is a strong performer in the rheumatoid arthritis market. With Pfizer’s loss of exclusivity for their blockbuster drug Lyrica, the next few years for could underperform for the company. Furthermore, if the drug is forced to be completely removed from the market, then these implications will be exacerbated. Whatever Pfizer chooses to do next will impact the success of Xeljanz.
References:
Baldock, D., Baynton, E., Baskett, A., & Bailey, N. (2018, October 25). The Future of JAK Inhibitors. Retrieved March 16, 2019, from http://www.pharmexec.com/future-jak-inhibitors
Bulik, B. S. (2019, February 19). Pfizer dethrones AbbVie as Xeljanz soars past Humira in January TV spending. Retrieved March 17, 2019, from https://www.fiercepharma.com/marketing/pfizer-dethrones-abbvie-as-xeljanz-soars-past-humira-as-leading-pharma-tv-spender-january
Cross, M., Smith, E., Hoy, D., Carmona, L., Wolfe, F., Vos, T., … & Buchbinder, R. (2014). The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Annals of the rheumatic diseases, 73(7), 1316-1322.
Crumly, J. (2019, February 20). Better Buy: Pfizer vs. Eli Lilly. Retrieved March 18, 2019, from https://www.fool.com/investing/2019/02/20/better-buy-pfizer-vs-eli-lilly.aspx
Drugs.com. (2012, November 6). FDA Approves Xeljanz for Rheumatoid Arthritis. Retrieved March 20, 2019, from https://www.drugs.com/newdrugs/fda-approves-xeljanz-rheumatoid-arthritis-3558.html
Drugs.com. (2016, February 26). Pfizer Announces FDA Approval of Xeljanz XR (tofacitinib citrate), the First and Only Once-Daily Oral JAK Inhibitor Treatment for Rheumatoid Arthritis. Retrieved March 16, 2019, from https://www.drugs.com/newdrugs/pfizer-announces-fda-approval-xeljanz-xr-tofacitinib-citrate-first-only-once-daily-oral-jak-4346.html
Drugs.com. (2017, December 14). Pfizer Announces FDA Approval of Xeljanz (tofacitinib) and Xeljanz XR for the Treatment of Active Psoriatic Arthritis. Retrieved from https://www.drugs.com/newdrugs/pfizer-announces-fda-approval-xeljanz-tofacitinib-xeljanz-xr-active-psoriatic-arthritis-4677.html
Drugs.com. (2018, May 30). Pfizer Announces U.S. FDA Approves Xeljanz (tofacitinib) for the Treatment of Moderately to Severely Active Ulcerative Colitis. Retrieved March 10, 2019, from https://www.drugs.com/newdrugs/pfizer-announces-u-s-fda-approves-xeljanz-tofacitinib-moderately-severely-active-ulcerative-colitis-4756.html
UCB Canada. (2019). Rheumatoid Arthritis. Retrieved March 16, 2019, from https://www.ucb-canada.ca/en/Patients/Conditions/Rheumatoid-Arthritis
Gadina, M. (2013, December). Janus kinases: an ideal target for the treatment of autoimmune diseases. In Journal of Investigative Dermatology Symposium Proceedings (Vol. 16, No. 1, pp. S70-S72). Elsevier.
Government of Canada, Health Canada. (2019, March 15). Clinical trial finds an increased risk of blood clots in the lungs and of death in rheumatoid arthritis patients taking high dose of tofacitinib (sold as Xeljanz or Xeljanz XR). Retrieved March 28, 2019, from https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2019/69336a-eng.php
FDA Biologics Evaluation and Research. (2018, September 18). Postmarketing Clinical Trials. Retrieved March 10, 2019, from https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Post-MarketActivities/Phase4Trials/default.htm
Helfand, C. (2018, May 22). Up-and-coming Pfizer, AbbVie drugs set to threaten Amgen, Celgene: Analyst. Retrieved February 24, 2019, from https://www.fiercepharma.com/marketing/pfizer-abbvie-jak-meds-set-to-threaten-amgen-celgene-share-analyst
Liu, A. (2019, March 01). EMA follows FDA in putting Pfizer’s Xeljanz under the microscope for safety concerns. Retrieved March 18, 2019, from https://www.fiercepharma.com/pharma/ema-follows-fda-s-suit-to-reassess-pfizer-s-xeljanz-over-safety-concerns
MacFarlane, L. A., & Todd, D. J. (2014). Kinase inhibitors: the next generation of therapies in the treatment of rheumatoid arthritis. International journal of rheumatic diseases, 17(4), 359-368.
Market Insider. (2018, January 10). JAK Inhibitors Expected to Introduce Fierce Competition to Treatment Paradigms Across a Wide Range of Autoimmune Indications, Including Psoriatic Arthritis, Atopic Dermatitis, Ulcerative Colitis,… | Markets Insider. Retrieved March 15, 2019, from https://markets.businessinsider.com/news/stocks/jak-inhibitors-expected-to-introduce-fierce-competition-to-treatment-paradigms-across-a-wide-range-of-autoimmune-indications-including-psoriatic-arthritis-atopic-dermatitis-ulcerative-colitis-1012837559
Market Realist. (2016, September 05). Why Pfizer’s Enbrel Faces a Sales Decline. Retrieved March 25, 2019, from https://articles.marketrealist.com/2016/08/why-pfizers-enbrel-faces-a-sales-decline/
Mayo Clinic. (2019, March 01). Rheumatoid arthritis. Retrieved March 16, 2019, from https://www.mayoclinic.org/diseases-conditions/rheumatoid-arthritis/symptoms-causes/syc-20353648
World Health Organization. (2016, November 17). Chronic rheumatic conditions. Retrieved March 14, 2019, from https://www.who.int/chp/topics/rheumatic/en/
Packer-Tursman, J. (2017, February 17). Pfizer’s Xeljanz falls short in match-up with Humira. Retrieved April 1, 2019, from https://www.biopharmadive.com/news/pfizers-xeljanz-falls-short-in-match-up-with-humira/436474/
Pfizer. (2012, November 6). U.S. Food And Drug Administration Approves Pfizer’s XELJANZ® (tofacitinib citrate) for Adults with Moderately to Severely Active Rheumatoid Arthritis (RA) Who Have Had an Inadequate Response or Intolerance to Methotrexate. Retrieved March 18, 2019, from https://www.pfizer.com/news/press-release/press-release-detail/u_s_food_and_drug_administration_approves_pfizer_s_xeljanz_tofacitinib_citrate_for_adults_with_moderately_to_severely_active_rheumatoid_arthritis_ra_who_have_had_an_inadequate_response_or_intolerance_to_methotrexate
Pfizer. (2018, October 26). Health Canada Approves New Indications for Xeljanz in Ulcerative Colitis (UC) & Psoriatic Arthritis (PsA). Retrieved March 17, 2019, from https://www.pfizer.ca/health-canada-approves-new-indications-xeljanztm-ulcerative-colitis-uc-psoriatic-arthritis-psa
Pfizer. (2019, February 19). Pfizer Announces Modification to Ongoing Tofacitnib FDA Post-Marketing Requirement Study in Patients with Rheumatoid Arthritis. Retrieved February 25, 2019, from https://investors.pfizer.com/investor-news/press-release-details/2019/Pfizer-Announces-Modification-to-Ongoing-Tofacitnib-FDA-Post-Marketing-Requirement-Study-in-Patients-with-Rheumatoid-Arthritis/default.aspx
Pfizer. (2019a, January 29). PFIZER REPORTS FOURTH-QUARTER AND FULL-YEAR 2018 RESULTS. Retrieved March 10, 2019, from https://investors.pfizer.com/investor-news/press-release-details/2019/PFIZER-REPORTS-FOURTH-QUARTER-AND-FULL-YEAR-2018-RESULTS/default.aspx
Pharmaceutical Technology. (2018a, June 13). Eli Lilly’s Olumiant: How will it far among JAK inhibitors? Retrieved March 17, 2019, from https://www.pharmaceutical-technology.com/comment/eli-lillys-olumiant-will-far-among-jak-inhibitors/
Pharmaceutical Technology. (2018, June 15). Pfizer’s blockbuster drug Lyrica comes off-patent in 2018. Retrieved March 10, 2019, from https://www.pharmaceutical-technology.com/comment/pfizers-blockbuster-drug-lyrica-comes-off-patent-2018/
Renauer, C. (2019, February 22). What Pharma Investors Need to Know About Pfizer’s Latest Disaster. Retrieved March 17, 2019, from https://www.fool.com/investing/2019/02/21/what-pharma-investors-need-to-know-about-pfizers-l.aspx
Taylor, P. (2019, March 29). Gilead and Galapagos close in on filing for Xeljanz rival. Retrieved April 1, 2019, from http://www.pmlive.com/pharma_news/gilead_and_galapagos_close_in_on_filing_for_xeljanz_rival_1282874
