20 Edge Therapeutics: Fast-tracking EG-1964 to Market or Not
Edge Therapeutics: Fast-tracking EG-1964 to Market or Not
By: Aman Razack
April 8th, 2018
On a surprisingly cold day at the tail end of March 2018, CEO of Edge Therapeutics Brian Leuthner and Senior Vice President and General Counsel Brad Middlekauff call in the rest of the management team for an emergency meeting. “I have called you all in today to discuss the future of the company,” said Brian. This statement raised concern for many of the other executives on the board. “We will soon have a 92% loss on our market capitalization and will need to lay off a large amount of employees”[1]. This information greatly shocked everyone in the room. “We need to figure out a short-term solution to appease our investors,” said Brad. “How could this drastic of a loss have happened in our stock” asked Liam Ratcliffe, a member of the Board of Directors. “We recently found issues with the side effects of our Phase III drug EG-1962,” stated Brian. This posed a huge problem, as the promise shown by EG-1962 was the reason for the major market cap of Edge. There Brian and the rest of the management team seemed to be vacillating on what to do now that their highly touted drug candidate is discontinuing its trials. “We must figure out a way to get our next most ready product, in this case EG-1964 through trials as effectively as possible,” said Brian.
As a someone who has over 25 years of experience in the market of hospital acute care, Brian is someone who has had to face adversity and tough situations before. Prior to being one of the Co-founders of Edge Therapeutics, he also served as the CEO to Fontus Pharmaceuticals, the Senior Head of Marketing for The Medicines Company and the Director of Market Development for ESP Pharma [2]. His sales and marketing jobs at Glaxo Wellcome and Ortho Biotech also seemed to help him thrive in leadership roles where he helped launch critical care products and strengthen customer relations. All in all, the situation he and Edge currently find themselves in will require as much experience and tenacity as possible.
Edge Therapeutics
Edge Therapeutics Inc. is a biotechnology company based in Berkeley Heights, New Jersey. It was founded in the 2009 by now CEO Brian Leuthner and the Chief Scientific Officer R. Loch Macdonald. The aim of Edge is to develop and commercialize therapies to be used for the treatment of acute, life-threatening neurological conditions. Specifically, they were looking into compounds that would be able to target rare neurological conditions for which current methods of treatment were insufficient [3].
As of 2017 the company contained thirty-eight employees [4], making them rather small in size, but what they lacked in size they made up for in experience, like Brian Leuthner. The Chief Operating Officer, Daniel Brennan was previously the Vice President of Lundbeck’s U.S. Neurology Business Unit and under his leadership he was able to launch four specialty products which caused the annual sales to grow from $60 million to $820 million. Senior Vice Preseident Brad Middlekauff also had great experience being the Chief Legal Officer at Kolltan Pharmaceuticals and the Senior Vice President and Medarex Inc [2].
With all of the experience from their executives, what Edge needed next was a novel product or process to break into this very saturated field. In June 2013, researchers brought forth very promising results with their microparticle formation named EG-1962, which is able to treat aneurysmal subarachnoid hemorrhaging in the brain [5]. Also, in October 2016, they filed for a trademark on a novel way to administer drugs to the specific site of injury, which they called the Precisa Platform™[6].
The only issue was that they were using a good portion of their funding in Research and Development for EG-1962 and also for General and Administrative purposes. They had used $34.3 million for R&D purposes related to EG-1962 clinical development and $17.7 million for G&A purposes related to personnel costs, investor relations cost and legal and professional fees, at the end of 2017. This totaled to a net loss of $50.9 million at the end of 2017 [7]. Although they incurred a net loss at the end of 2017 and 2016, Edge still had $47.4 million in their reserves for any other costs
Aneurysmal Subarachnoid Hemorrhage (aSAH)
Subarachnoid hemorrhages are a very serious type of stroke that occurs in the subarachnoid space in the brain. It is caused by a ruptured brain aneurysm or a head injury. In a healthy brain, the subarachnoid space in between the brain and the skull contains cerebrospinal fluid, however, when an aneurysm occurs, blood is released into this space surrounding the arteries which increases pressure on the brain, damaging the brain cells [8]. Also, the artery that was affected is not able to transport oxygenated blood to the specific area of the brain that it was leading to, causing there to be a deprivation of blood, leading to a stroke and ultimately death of brain tissue. This process can be seen in Exhibits 1 and 2.
The amount of people affected each year by aSAH is growing at a staggering rate, especially in countries in the Western Hemisphere. Looking at the United States, there are about 30 000 people who suffer from a brain aneurysm each year, where about 40% are fatal. It has also been shown that women are more likely to suffer from brain aneurysms than men and that African American’s and Hispanics are more susceptible than Caucasians. In addition, across the world there are approximately 500 000 deaths each year from brain aneurysms with most of the victims below the age of 50 [9]. One of the most debilitating facts however is that of the people who do recover, about 57% will end up with a disability, causing lay offs from work.
There are a few specific ways which aSAH is diagnosed: the first being the use of Computed Tomography angiography (CTA) which constitutes the injection of a specific contrasting compound into the blood stream allowing the arteries in the brain. Another way is by lumbar puncture where a needle is inserted into the subarachnoid space of the spinal canal to determine if blood is in the cerebral spinal fluid. This procedure is usually performed as a secondary test if a regular CT scan does not show bleeding. The third way to diagnose aSAH is by Magnetic Resonance Imaging (MRI), which uses a magnetic field and radio frequency waves to give a detailed view of the soft tissues in the brain [8].
Current Surgical Treatments Available for aSAH
With the rise of aSAH and other stroke related illnesses, there will also be a rise in treatments to go along with it. It has been predicted that the cost of therapeutic technology relating to stroke and other brain diseases will be around $323 million with the treatment of acute stroke management estimated to be $1.5 billion, between 2015-2019. According to the report, “Emerging Global Market for Neurointerventional Technologies in Stroke” by Patrick Driscoll of MedMarket Diligence,
“Stroke is associated with costly long-term care, especially for a patient population that is typically older and more susceptible to its complications, but neurointerventional treatment have succeeded in both making a positive clinical impact and securing respectable revenue streams for manufacturers [10].
With that being said, one of the current treatments is the use of surgery. One of the surgical procedures that is used is surgical clipping, which involves opening the skull at the spot of the aneurysm and then inserting a titanium clip around the neck to prevent the blood flow from entering. Another procedure is endovascular coiling, which involves the insertion of a catheter into an artery in the groin that is sent to the spot of the blood vessel burst, where titanium coils or liquid glue is packed into the aneurysm to prevent blood from entering [8]. The control of vasospasm is also of importance as it leads to the narrowing of the arteries and decreased blood flow in the area of the brain which the artery feeds (Exhibit 2). The monitoring of vasospasms is performed by a Transcranial Doppler (TCD) to measure the blood flow of the arteries (Exhibit 3) and can determine which arteries show vasospasm as well as the severity. The effects of vasospasm can lead to delayed cerebral ischemia (DCI), the restriction of blood flow, causing parts of the brain to die.
Current Pharmaceuticals on the Market
As can be inferred however, these procedures are highly invasive and would not be preferred by patients. Because of this, there has been the development of several drugs one of which, called nimodipine, has shown to have very positive effects on the treatment of vasospasm.
Bayer: Nimotop
Originally the drug was discovered and tested in 1983 by a group of scientists on a series of patients to determine whether or not nimodipine would reduce the severity of ischemic neurological deficits [11]. What they found was that the 56 patients given the drug did not suffer any worse neurological outcomes compared to the patients given a placebo.
This product was then developed into an oral pill by the pharmaceutical giant Bayer and was granted FDA approval in 2005. Called Nimotop®, the drugs’ usage was to improve neurological outcome by reducing the severity and incidence of ischemic deficits by subarachnoid hemorrhaging. Specifically, this drug was known as a calcium channel blocker. During contraction of the muscle tissue, calcium ions are needed to enter the muscle cells through depolarization, creating small currents ultimately causing the muscles to contract. By administering nimodipine, calcium ion transfer is inhibited, thus inhibiting the contraction of the muscles in the blood vessels [12].
The effect of nimodipine was shown to be very useful in patients who suffered from vasospasms but it also came along with several side effects such as: heart failure, vomiting and neurological deterioration to name a few [12]. This led to the development of drugs by other companies.
Pfizer: Calan
One of the competitors of Nimotop was Calan® and worked in a similar way to nimodipine. The name of the compound used in Calan is verapamil and it is also a calcium ion inhibitor. The way it works is by dialating the arteries in normal and ischemic regions thus increasing the amount of oxygenated blood that is delivered in patients with arterial spasms [13]. Although verapamil was mostly used for the treatment of vasospasm related to the heart, as what is was originally marketed for in 1984, it has recently been shown to be beneficial for treatment of cerebral vasospasm [14].
This however came along with some controversy in its use in cerebral vasospasm. One study conducted found there was a significant reduction in arterial blood pressure after inter-arterial injection14. There is also not enough research to prove that verapamil has a large clinical application.
Amgen: Epogen
A third therapeutic on the market is the use of the erythropoietin Epogen. This product is different from the two previously described since it uses glycoprotein instead of a chemical compound. For the past 30 years Amgen has been producing Epogen specifically for dialysis patients and has been splitting the erythropoietin (EPO) market with Johnson & Johnson [15]. This market has been a growing one as well; it is expected that it will reach $11.9 billion by 2020 with a solid CAGR of 9.7% from 2014 to 2020 [16]. One of the reasons for this high growth is the fact that EPO drugs are used in Cancer and HIV patients who suffer from anemia after chemotherapy, and EPO’s are used treat this.
In some early animal experiments, EPO’s have also been shown to have a neuroprotective role in cerebral ischaemia. The mechanism for their action in decreasing vasospasm is not clearly understood however. Some proposed mechanisms are its effect to limit inflammation, stop cell death in surrounding tissue or upregulation of neuron production [14].
Even though there is a lot of potential for the adoption of EPO drugs, they are still only in animal trials for its use in vasospasms. There is still much work to be done, especially the use of large clinical trials, before this can be used as a potential treatment.
EG-1962
With all of this information on the market, Brian, Brad and the rest of the team wanted to figure out a way to administer an anti-vasospasm drug without the side effects of other drugs and procedures. With several years of research they were able to engineer a specific development platform which they coined the Precisa Platform. Precisa is a programmable, biodegradable, polymer-based therapeutic that is able to deliver compounds directly to the site of injury, which would potentially prevent side effects from other drugs [17]. To be specific, a therapeutic, is adhered to the surface of the Precisa microparticles and then administered via brain catheter. The microparticle is then able to release the therapeutic in a controlled manner, allowing for a one-time administration [Exhibit 4]. The polymer made up of poly (DL-Lactic-co-glycolide) (PLGA) is then broken down into lactic acid, a naturally occurring product in the body.
The entire system composed of the Precisa microparticle and nimodipine makes up the EG-1962 product. As mentioned before, the current method of oral or intravenous dosing is known to cause serious side effects, one of the more serious is hypotension where blood flow is drastically decreased in the blood vessels. For this reason EN-1962 was made to deliver nimodipine to the brain as one single dose with drug exposure lasting 21-days, while also avoiding the dose-limiting side effects [17].
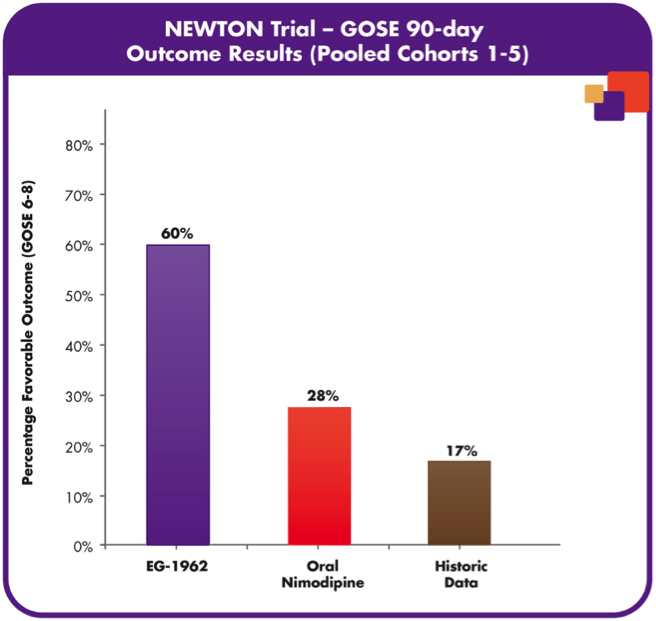
The previous animal trials were so promising that the United States FDA and European Commission both granted orphan drug designation for the treatment of aSAH. In the North American NEWTON phase 1/2 study, the tolerability and pharmacokinetics of EG-1962 was measured with the therapeutic administered via external ventricular drain (Exhibit 5) to 72 patients. What was observed after 90 days was that EG-1962 met all of the endpoints for safety, pharmacokinetics and tolerability and also 60% of the patients treated with the therapeutic showed favourable outcomes compared to the 28% administered oral nimodipine (Exhibit 6) [17]. Furthermore, the reduction in vasospasm, delayed cerebral ischemia, reduction in rescue therapies and shorter ICU times were all shown after the use of EG-1962, with no hypotension. With Phase 1 and 2 trials so successfully, EG-1962 looked like it was going to give extremely promising results in Phase 3, however this was not the case. It was determined that the Phase 3 study demonstrated a low probability of achieving statistically-significant difference compared to the standard care and was suggested to stop the study by the Data Monitoring Committee [18]. Although bad news, there were still some options that Brain and the rest of the management team had.
Possibility
Although their main product seemed to be bust, Edge still had another product on deck, EG-1964. This therapeutic would again aid in neurological damage but more specifically it would be used to treat subdural hemotomas (SDH). SDH is the collection of blood on the outer surface of the brain and is usually caused by head injury. While this is not yet a leading neurological disorder, it is predicted that by 2030 there will be at least 60 000 people who suffer from SDH in the US alone [19]. However, the production and phase testing of EG-1964 still looks very far off.
This leads to Edge’s most valuable product, its Precisa technology. The advantage of this type of technology is the fact that any compound is able to adhere to its surface; the microparticle just needs to be engineered so that the polymer-based formulations are adapted to the proper size, surface property, dose level and release profile [17]. With this type of technology, Edge could use other drugs with Precisa to administer drugs quicker and to their specified target more accurately. Moreover, the release rates of the drugs would be timed accurately thus making patients not have to worry about taking their drugs at specific times or over and underdosing. With this trademark, Edge will still be able to compete in the therapeutic industry, it however might take a little time.
The Restart
As Brian and the rest of the team sat in the conference room, they continued to mull over whether bringing EG-1964 to market would be the best idea. “I think the best idea would be to see how Eg-1964 does in its Phase 1 trials and not push it out as soon as possible,” said Brad MiddleKauff. Looking around the table and observing the unanimous head nods, Brian then stated, “Now that we all agree on that, lets see what we can do about Precisa.” The team then proceeded to brainstorm.
Appendix
Exhibit 1: Aneurysmal Subarachnoid Hemorrhage (aSAH). The blood from the ruptured artery fills the subarachnoid space forming a blot clot and increasing pressure [8].
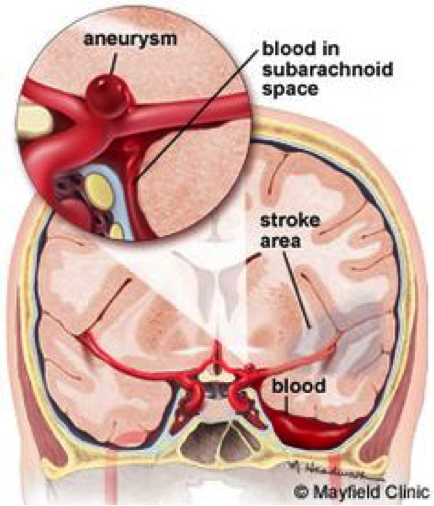
Exhibit 2: Diagram displaying the subsequent affects of a subarachnoid hemorrhage on one area of the brain [3].
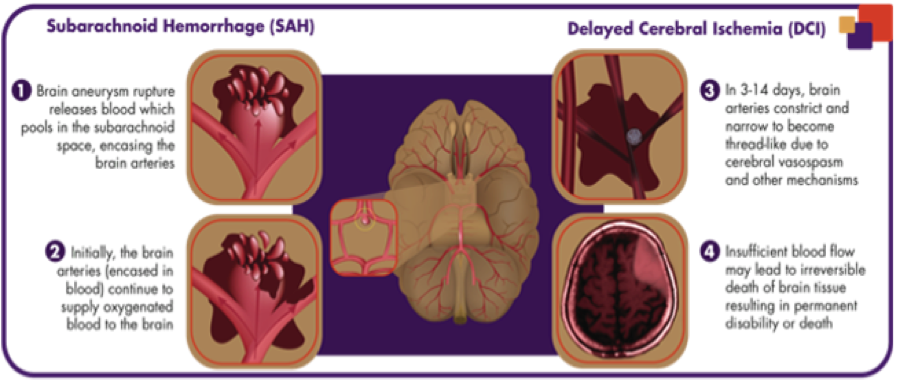
Exhibit 3: Transcranial Doppler probe (TCD) using ultrasound technology to examine cerebral arteries, looking for blood flow and diagnosing vasospasm [8].
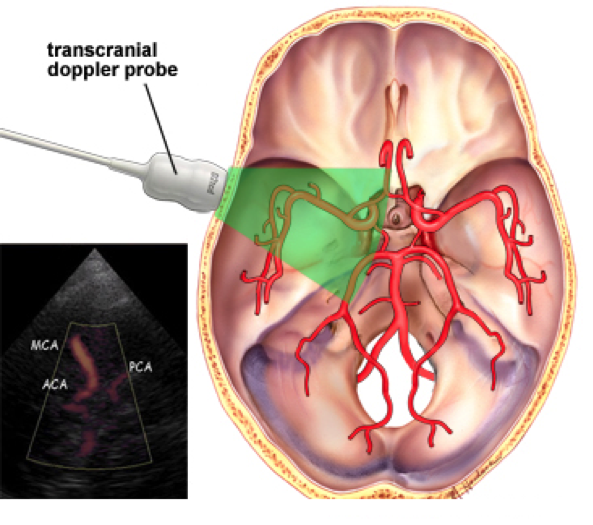
Exhibit 4: EG-1962 made up of Precisa microparticles and nimodipine showing the release of the drug and degradation of the polymer [3].
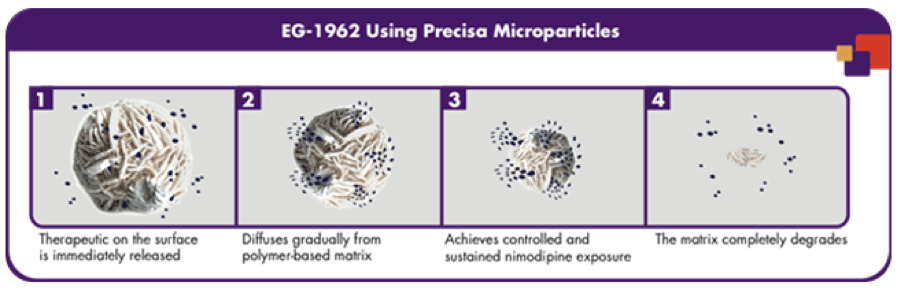
Exhibit 5: Depiction of external ventricular drain used to administer EG-1962 to specific area of the brain. Red arrows indicate areas where blood flows after aneurysm [3].
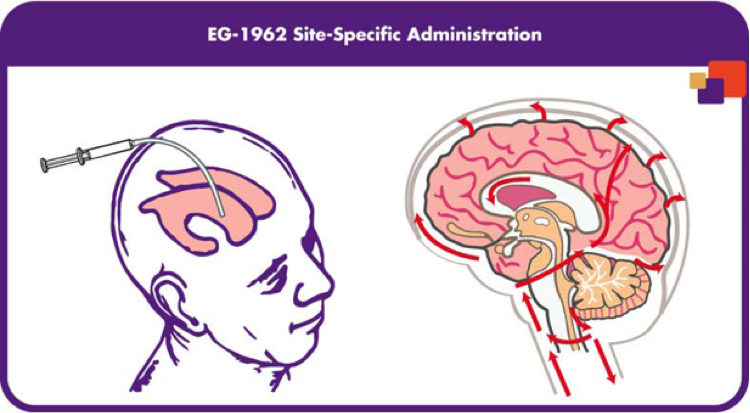
Exhibit 6: Percentage of favourable outcomes for EG-1962 and other drugs on 72 patients for 90 days [3].
Endnotes
1 Chatsko, M. (2018, March 28). Here’s Why Edge Therapeutics Stock Collapsed Today. Retrieved April 11, 2018, from https://finance.yahoo.com/news/apos-why-edge-therapeutics-stock-165311305.html
2 Edge Therapeutics, Inc. (n.d.). Retrieved April 11, 2018, from http://www.edgetherapeutics.com/about/#clinical-advisors
3 Edge Therapeutics, Inc. (n.d.). Retrieved April 11, 2018, from http://www.edgetherapeutics.com/about/
4 Financial Times: Edge Therapeutics Inc. (n.d.). Retrieved April 11, 2018, from https://markets.ft.com/data/equities/tearsheet/profile?s=EDGE:NSQ
5 Edge Therapeutics Reports Positive Early Clinical Experience with EG-1962 to Prevent Serious Complications after Brain Hemorrhage. (2013, June 27). Retrieved April 11, 2018, from https://www.businesswire.com/news/home/20130627005590/en/Edge-Therapeutics-Reports-Positive-Early-Clinical-Experience
6 PRECISA PLATFORM Trademark Application of Edge Therapeutics Inc. – Serial Number 87196078 :: Justia Trademarks. (n.d.). Retrieved April 11, 2018, from https://trademarks.justia.com/871/96/precisa-87196078.html
7 Edge Therapeutics Reports Fourth Quarter and Full Year 2017 Financial Results. (n.d.). Retrieved April 11, 2018, from https://www.nasdaq.com/press-release/edge-therapeutics-reports-fourth-quarter-and-full-year-2017-financial-results-20180301-00309
8 Mayfield Brain & Spine. (n.d.). Subarachnoid hemorrhage & vasospasm. Retrieved April 11, 2018, from https://www.mayfieldclinic.com/PE-SAH.HTM
9 Brain Aneurysm Statistics and Facts. (n.d.). Retrieved April 11, 2018, from https://www.bafound.org/about-brain-aneurysms/brain-aneurysm-basics/brain-aneurysm-statistics-and-facts/
10 USA Archives – © 2018, MedMarket Diligence, LLC. (n.d.). Retrieved April 11, 2018, from https://blog.mediligence.com/tag/usa/
11 Cerebral Arterial Spasm – A Controlled Trial of Nimodipine in Patients with Subarachnoid Hemorrhage | NEJM. (n.d.). Retrieved April 11, 2018, from http://www.nejm.org/doi/full/10.1056/NEJM198303173081103
12 Katz, R. (2006, January 20). Nimotop (nimodipine): FDA Approved Labeling Text. Retrieved April 8, 2018, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018869s014lbl.pdf
13 CALAN® SR (verapamil hydrochloride) Sustained-Release Oral Caplets. (n.d.). Retrieved April 11, 2018, from http://labeling.pfizer.com/ShowLabeling.aspx?id=534
14 Qiu, Y. L., & Jiang, J. S. (2016, February 18). Drug treatment of cerebral vasospasm after subarachnoid hemorrhage following aneurysms. Retrieved April 10, 2018, from https://cnjournal.biomedcentral.com/articles/10.1186/s41016-016-0023-x
15 Long, P. G. (2013, May 02). EPO Pills: A Challenge For Amgen And Johnson & Johnson. Retrieved April 11, 2018, from https://seekingalpha.com/article/1394161-epo-pills-a-challenge-for-amgen-and-johnson-and-johnson
16 Global Erythropoietin Drugs Market 2013 – 2020: EPO market to reach $11.9 billion by 2020. (n.d.). Retrieved April 11, 2018, from https://www.prnewswire.com/news-releases/global-erythropoietin-drugs-market-2013—2020-epo-market-to-reach-119-billion-by-2020-300038976.html
17 Pipeline. (n.d.). Retrieved April 11, 2018, from http://www.edgetherapeutics.com/pipeline/
18 Press Release. (n.d.). Retrieved April 11, 2018, from http://investors.edgetherapeutics.com/phoenix.zhtml?c=253911&p=irol-newsArticle&ID=2340075
19 Balser, D., Farooq, S., Mehmood, T., Reyes, M., & Samadani, U. (2015, November). Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. Retrieved April 11, 2018, from https://www.ncbi.nlm.nih.gov/pubmed/25794342
