2.1 Systems of Measurement
Learning Objectives
By the end of this section, you will be able to:
- Name the units in the Metric system (SI) and recognize their prefixes and their values.
- Perform metric-to-metric unit conversions using the decimal point method.
- Perform metric-to-metric unit conversions using dimensional analysis.
- Add and subtract SI units.
- Know and use the relationship between mL, g, and, cm3.
- Recognize the U.S. system of units.
- Perform U.S. system-to-U.S. system conversions using dimensional analysis.
- Perform unit conversions (from any system) using dimensional analysis.
- Convert between Fahrenheit and Celsius temperatures.
Measurement Systems
The Metric System
Metric system (SI – international system of units): the most widely used system of measurement in the world. It is based on the basic units of meter, kilogram, second, etc.
In the metric system, units are related by powers of 10. The roots words of their names reflect this relation. For example, the basic unit for measuring length is a meter. One kilometer is 1,000 meters; the prefix kilo means thousand. One centimeter is [latex]\frac{1}{100}[/latex] of a meter, just like one cent is [latex]\frac{1}{100}[/latex] of one dollar.
SI common units:
| Quantity |
Unit | Unit Symbol |
|---|---|---|
| Length | meter | m |
| Mass (or weight) | gram | kg |
| Volume | litre | L |
| Time | second | s |
| Temperature | degree (Celsius) | °C |
Metric prefixes (SI prefixes): large and small numbers are made by adding SI prefixes, which is based on multiples of 10.
Metric conversion table:
| Prefix | Symbol (abbreviation) | Power of 10 | Multiple value | Example |
|---|---|---|---|---|
| giga | G | 109 | 1,000,000,000 | 1 Gm = 1,000,000,000 m |
| mega | M | 106 | 1,000,000 | 1 Mm = 1,000,000 m |
| kilo- | k | 103 | 1,000 | 1 km = 1,000 m |
| hecto- | h | 102 | 100 | 1 hm = 100 m |
| deka- | da | 101 | 10 | 1 dam = 10 m |
| meter/gram/litre | 1 (100) | |||
| deci- | d | 10-1 | 0.1 | 1 m = 10 dm |
| centi- | c | 10-2 | 0.01 | 1 m = 100 cm |
| milli- | m | 10-3 | 0.001 | 1 m = 1,000 mm |
| micro | µ or mc | 10-6 | 0.000 001 | 1 m = 1,000,000 µm |
| nano | n | 10-9 | 0.000 000 001 | 1 m = 1,000,000,000 nm |
| pico | p | 10-12 | 0.000 000 000 001 | 1 m = 1,000,000,000,000pm |
A good way to remember the order of the metric prefixes is by using a mnemonic device such as “Great Mighty King Henry died by drinking chocolate malted milk not poison”. Notice that the first letter of each word reminds you of the metric prefix, and the word “by” represents the base units. Feel free to use this particular mnemonic device, or come up with your own!
Metric prefix for length, weight and volume:
| Prefix | Length (m – meter) | Weight (g – gram) | Liquid volume (L – litre) |
|---|---|---|---|
| giga (G) | Gm (Gigameter) | Gg (Gigagram) | GL (Gigalitre) |
| mega (M) | Mm (Megameter) | Mg (Megagram) | ML (Megalitre) |
| kilo (k) | km (Kilometer) | kg (Kilogram) | kL (Kilolitre) |
| hecto (h) | hm (hectometer) | hg (hectogram) | hL (hectolitre) |
| deka (da) | dam (dekameter) | dag (dekagram) | daL (dekalitre) |
| meter/gram/litre | m (meter) | g (gram) | L (litre) |
| deci (d) | dm (decimeter) | dg (decigram) | dL (decilitre) |
| centi (c) | cm (centimeter) | cg (centigram) | cL (centilitre) |
| milli (m) | mm (millimeter) | mg (milligram) | mL (millilitre) |
| micro (µ or mc) | µm or mcm (micrometer) | µg or mcg (microgram) | µL or mcL (microlitre) |
| nano (n) | nm (nanometer) | ng (nanogram) | nL (nanolitre) |
| pico (p) | pm (picometer) | pg (picogram) | pL (picolitre) |
The more commonly used equivalencies of measurements in the metric system are shown in Table 2.1.4. The common abbreviations for each measurement are given in parentheses. Please note, that you will need to be able to convert the units outside of this table as well.
Metric System of Measurement
| Length | Mass | Capacity |
|---|---|---|
| 1 kilometer (km) = 1,000 m 1 hectometer (hm) = 100 m 1 dekameter (dam) = 10 m 1 meter (m) = 1 m 1 decimeter (dm) = 0.1 m 1 centimeter (cm) = 0.01 m 1 millimeter (mm) = 0.001 m |
1 kilogram (kg) = 1,000 g 1 hectogram (hg) = 100 g 1 dekagram (dag) = 10 g 1 gram (g) = 1 g 1 decigram (dg) = 0.1 g 1 centigram (cg) = 0.01 g 1 milligram (mg) = 0.001 g |
1 kiloliter (kL) = 1,000 L 1 hectoliter (hL) = 100 L 1 dekaliter (daL) = 10 L 1 liter (L) = 1 L 1 deciliter (dL) = 0.1 L 1 centiliter (cL) = 0.01 L 1 milliliter (mL) = 0.001 L |
| 1 meter = 100 centimeters 1 meter = 1,000 millimeters |
1 gram = 100 centigrams 1 gram = 1,000 milligrams |
1 liter = 100 centiliters 1 liter = 1,000 milliliters |
Performing Metric to Metric Conversions
One of the most convenient things about the metric system is that we can use its decimal nature to convert from one unit to the other simply by moving the decimal point to the left or to the right.
Steps for metric conversion:
- Identify the number of places to move the decimal point.
– Convert a smaller unit to a larger unit: move the decimal point to the left.
– Convert a larger unit to a smaller unit: move the decimal point to the right.
Example 2.1.1
326 mm = (?) m
Solution
Step 1: Identify mm (millimeters) and m (meters) on the conversion table.
Step 2: Count places from mm to m:
3 places left
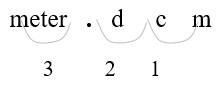
Step 3: Move 3 decimal places.
Convert a smaller unit (mm) to a larger (m) unit: move the decimal point to the left.
[latex](1 m = 1000 mm)[/latex]
Step 4: Move the decimal point three places to the left.
[latex]326. mm = 0.326 m[/latex]
Example 2.1.2
4.675 hg = (?) g
Solution
Step 1: Identify hg (hectograms) and g (grams) on the conversion table.
Step 2: Count places from hg to g:
2 places right
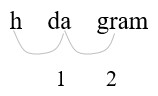
Step 3: Move 2 decimal places.
[latex](1 hg = 100 g)[/latex]
Step 4: Convert a larger unit (hg) to a smaller (g) unit.
Move the decimal point to the right.
Step 5: Move the decimal point two places to the right.
[latex]4.765 hg = 476.5 g[/latex]
Try It
1) Convert 0.2744kg to micrograms.
Solution
274,400,000 mcg (or 274,400,00 µg)
2) Convert 12,940,000 nL to decilitres.
Solution
0.1294 dL
Being able to convert units by shifting the decimal point left or right is convenient and does work for a lot of our metric to metric conversions. However, as we will see later in the section, converting more complex units may be confusing if we are using the decimal point method. Thus, it is important to have an understanding of the technique of Dimensional Analysis (or the Unit Factor Method).
Dimensional Analysis (or the Unit Factor Method)
Convert units using the dimensional analysis or (the Unit Factor Method)
Step 1: Write the original term as a fraction (over 1).
Example: 10g can be written as [latex]\frac{10g}{1}[/latex]
Step 2: Write the conversion formula as a fraction, [latex]\frac{1}{( )}[/latex] or [latex]\frac{( )}{1}[/latex]
Example: 1m = 100 cm can be written as [latex]\frac{1m}{(100cm)}[/latex] or [latex]\frac{(100cm)}{1m}[/latex]
Step 3: Put the desired or unknown unit on the top.
Step 4: Multiply the original term by [latex]\frac{1}{( )}[/latex] or [latex]\frac{( )}{1}[/latex] (Cancel out the same units)
Example 2.1.3
1200 g = (?) kg
Solution
Step 1: Write the original term (the left side) as a fraction.
[latex]1200 g = \frac{1200 g}{1}[/latex]
Step 2: Write the conversion formula as a fraction.
“kg” is the desired unit.
[latex]1 kg = 1000g:\frac{1 kg}{(1000 g)}[/latex]
Step 3: Multiply.
The units “g” cancel out.
[latex]\begin{eqnarray*}1200 g &=& \frac{1200 \bcancel{g}}{1} \times{\frac{1 kg}{(1000 \bcancel{g})}}\\&=& \frac{1200 kg}{1000}\\&=& 1.2 kg\end{eqnarray*}[/latex]
Example 2.1.4
30 cm = (?) mm
Solution
Step 1: Write the original term (the left side) as a fraction.
[latex]30 cm = \frac{30 cm}{1}[/latex]
Step 2: Write the conversion formula as a fraction.
“mm” is the desired unit.
[latex]1 cm = 10 mm: \frac{(10 mm)}{1cm}[/latex]
Step 3: Multiply.
The units “cm” cancel out.
[latex]\begin{eqnarray*}30 cm &=& \frac{30 \bcancel{cm}}{(1 mm)} \times{\frac{(10 mm)}{1 \bcancel{cm}}}\\&=& \frac{(30)(10) mm}{1}\\&=& 300 mm\end{eqnarray*}[/latex]
Try It
Use dimensional analysis to convert the following units:
3) Convert 28.4 dag to g.
Solution
284 g
4) Convert 0.00485kL to dL.
Solution
48.5 dL
Example 2.1.5
Have you ever run a 5K or 10K race? The length of those races are measured in kilometers. The metric system is commonly used in the United States when talking about the length of a race.
Nick ran a 10K race. How many meters did he run?
Solution
We will convert kilometers to meters using the identity property of multiplication.
Step 1: Multiply the measurement to be converted by 1.
[latex]10\;\text{kilometers}\;\times\;1[/latex]
Step 2: Write 1 as a fraction relating kilometers and meters.
[latex]10\;\text{kilometers}\cdot\frac{1,000\;\text{meters}}{1\;\text{kilometers}}[/latex]
Step 3: Simplify.
[latex]\frac{10\;\cancel{\text{kilometers}}\cdot1,000\;m}{1\;\cancel{\text{kilometer}}}[/latex]
Step 4: Multiply.
[latex]10,000[/latex] meters. Nick ran [latex]10,000[/latex] meters.
Try It
5) Sandy completed her first 5K race! How many meters did she run?
Solution
5,000 meters
6) Herman bought a rug 2.5 meters in length. How many centimeters is the length?
Solution
250 centimeters
Example 2.1.6
Eleanor’s newborn baby weighed 3,200 grams. How many kilograms did the baby weigh?
Solution
We will convert grams into kilograms.
Step 1: Multiply the measurement to be converted by 1.
[latex]3,200\;\text{grams}\;\times\;1[/latex]
Step 2: Write 1 as a function relating kilograms and grams.
[latex]3,200\;\text{grams}\cdot\frac{1kg}{1,000\;\text{grams}}[/latex]
Step 3: Simplify.
[latex]3,200\;\cancel{\text{grams}}\;\cdot\;\frac{1kg}{1,000\;\cancel{\text{grams}}}[/latex]
Step 4: Multiply.
[latex]\frac{3,200\;\text{kilograms}}{1,000}[/latex]
Step 5: Divide.
[latex]3.2[/latex] kilograms. The baby weighed [latex]3.2[/latex] kilograms.
Try It
7) Kari’s newborn baby weighed 2,800 grams. How many kilograms did the baby weigh?
Solution
2.8 kilograms
8) Anderson received a package that was marked 4,500 grams. How many kilograms did this package weigh?
Solution
4.5 kilograms
Example 2.1.7
Samadia took 800mg of Ibuprofen for her inflammation. How many grams of Ibuprofen did she take?
Solution
We will convert milligrams to grams using the identity property of multiplication.
Step 1: Multiply the measurement to be converted by 1.
[latex]800\;\text{milligrams}\times1[/latex]
Step 2: Write 1 as a fraction relating kilometres and metres.
[latex]800\;\text{milligrams}\times\frac{1\;\text{gram}}{1000\;\text{milligrams}}[/latex]
Step 3: Simplify.
[latex]800\;\text{milligrams}\times\frac{1\;\text{gram}}{1000\;\text{milligrams}}[/latex]
Step 4: Multiply.
[latex]0.8\;\text{grams}[/latex]
Samadia took [latex]0.8[/latex] grams of Ibuprofen.
Example 2.1.8
Dena’s recipe for lentil soup calls for 150 milliliters of olive oil. Dena wants to triple the recipe. How many liters of olive oil will she need?
Solution
We will find the amount of olive oil in millileters then convert to liters.
Step 1: Translate to algebra.
[latex]3\times{150}[/latex]
Step 2: Multiply.
[latex]450 mL[/latex]
Step 3: Convert to liters.
[latex]450 mL\times{\frac{0.001L}{1 mL}}[/latex]
Step 4: Simplify.
[latex]0.45 L[/latex]
Dena needs 0.45 liters of olive oil.
Try It
9) Klaudia took 0.125 grams of Ibuprofen for his headache. How many milligrams of the medication did she take?
Solution
125 milligrams
10) A recipe for Alfredo sauce calls for 250 milliliters of milk. Renata is making pasta with Alfredo sauce for a big party and needs to multiply the recipe amounts by 8. How many liters of milk will she need?
Solution
2 liters
11) To make one pan of baklava, Dorothea needs 400 grams of filo pastry. If Dorothea plans to make 6 pans of baklava, how many kilograms of filo pastry will she need?
Solution
2.4 kilograms
Example 2.1.9
The volume of blood coursing throughout an adult human body is about 5 litres. Convert it to millilitres.
Solution
We will convert litres to millilitres. In the Metric System of Measurement table, we see that 1 litre = 1,000 millilitres.
Step 1: Multiply by 1, writing 1 as a fraction relating litres to millilitres.
[latex]5 L\times{\frac{1000 mL}{1L}}[/latex]
Step 2: Simplify.
[latex]5\cancel L\times\frac{1000mL}{1\cancel L}\;=\;5\;\times\;1000mL[/latex]
Step 3: Multiply.
[latex]5000 mL[/latex]
As we saw before, when we are converting metric to metric units, you may see a pattern. Since the system is based on multiples of ten, the calculations involve multiplying by multiples of ten. We have learned how to simplify these calculations by just moving the decimal.
Remember that to multiply by 10, 100, or 1,000, we move the decimal to the right one, two, or three places, respectively. To multiply by 0.1, 0.01, or 0.001, we move the decimal to the left one, two, or three places, respectively.
We can apply this pattern when we make measurement conversions in the metric system. In Figure 2.1.1 , we changed 3,200 grams to kilograms by multiplying by [latex]\frac{1}{1000}[/latex] (or 0.001). This is the same as moving the decimal three places to the left.
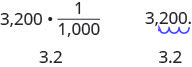
Example 2.1.10
Convert:
a. 350 L to kiloliters
b. 4.1 L to milliliters.
Solution
a. We will convert liters to kiloliters. In Table 2.1.4, we see that 1 kiloliter= 1,000 liters.
Step 1: Multiply by 1, writing 1 as a fraction relating liters to kiloliters.
[latex]350 L\cdot\frac{1 kL}{1,000 L}[/latex]
Step 2: Simplify.
[latex]350\;\cancel L\;\cdot\;\frac{1kL}{1,000\;\cancel L}[/latex]
Step 3: Move the decimal 3 units to the left.
[latex]0.35 kL[/latex]
b. We will convert liters to milliliters. From Table 2.1.4 we see that 1 liter=1,000 milliliters.
Step 1: Multiply by 1, writing 1 as a fraction relating liters to milliliters.
[latex]4.1 L\cdot\frac{1,000 mL}{1 L}[/latex]
Step 2: Simplify.
[latex]4.1\;\cancel L\;\cdot\;\frac{1,000 mL}{1,000\;\cancel L}[/latex]
Step 3: Move the decimal 3 units to the right.
[latex]{4}{\color{red}{\overrightarrow.}}{1_{\color{red}{1}}0_{\color{red}{2}}0_{\color{red}{3}}\;=\;4,100{\color{red}{.}}{\color{red}{\;}}{\color{black}{m}}}{\color{black}{L}}[/latex]
Try It
12) Convert:
a. 725 L to kiloliters
b. 6.3 L to milliliters
Solution
a 7,250 kiloliters
b 6,300 milliliters
13) Convert:
a. 350 hL to liters
b. 4.1 L to centiliters
Solution
a 35,000 liters
b 410 centiliters
As we see, even when doing dimensional analysis, we can use the pattern of multiplying by powers of ten and shift our decimal point to the left or right accordingly to find our answers and make our calculations more simple. However, what might we do if we needed to convert from milligrams per decilitre to grams per litre. When we use these types of units, it can make it more difficult to simply move the decimal point to the left and right. In the following example, we see how dimensional analysis can help us stay organized and convert these types of units.
Example 2.1.11
100 m/s = (?) km/h
Solution
Step 1: Write the original term (the left side) as a fraction.
[latex]100 m/s=\frac{100m}{1s}[/latex]
Step 2: Write the conversion formulas required as fractions.
“km/h” is the desired unit
[latex]1000m=1km\;\text{and}\;1h=3600s[/latex]
[latex]\frac{1km}{1000m}\;\text{and}\;\frac{3600s}{1h}[/latex]
Step 3: Multiply.
The units “m” and “s” cancel out.
[latex]\begin{align*} 100m/s&=\frac{100\cancel m}{1\cancel s}\times\frac{1km}{1000\cancel m}\times\frac{3600\cancel s}{1h}\\ &=\frac{100\times3600km}{1\times1000h}\\ &=360km/h \end{align*}[/latex]
Try It
14) Convert 0.000005kg/L to micrograms per decilitre.
Solution
500 mcg/dL or 500 mu g/dL.
Adding and subtracting SI measurements:
Example 2.1.12
Combine after converting to the same unit.
a. [latex]\begin{align*} 3m\;\;\;&\\ \underline{-2000mm}& \end{align*}[/latex]
b.[latex]\begin{align*} 25kg&\\ \underline{ \;\;\;+4g}& \end{align*}[/latex]
Solution
a.
Step 1: Convert to the same unit.
[latex]1 m = 1,000 mm[/latex]
Step 2: Subtract.
[latex]\begin{align*} 3000mm&\\ \underline{-2000mm}&\\ 1000mm& \end{align*}[/latex]
b.
Step 1: Convert to the same unit.
[latex]1 kg = 1000 g[/latex]
Step 2: Add.
[latex]\begin{align*} 25000g&\\ \underline{ \;\;\;\;\;+4g}&\\ 25004g& \end{align*}[/latex]
The Relationship between mL, g, and cm3
How are mL, g, and cm3 related?
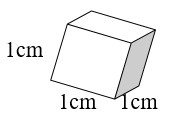
- A cube takes up 1 cm3 of space (1 cm × 1 cm × 1 cm = 1cm3).
- A cube holds 1 mL of water and has a mass of 1 gram at 4°C.
- 1 cm3 = 1 mL = 1 g
Example 2.1.13
Convert.
a. 16cm3 = ( ? ) g
b. 9 L = ( ? ) cm3
c. 35 cm3 = (?) cL
d. 450 kg = (?) L
Solution
a. 16cm3 = ( ? ) g
Step 1: Covert cm3 to g.
[latex]\begin{eqnarray*}1\;cm^3\;&=&\;\;1g\\16\;cm^3\;&=&\;16g\end{eqnarray*}[/latex]
b. 9 L = ( ? ) cm3
Step 1: Convert L to mL.
[latex]\begin{eqnarray*}1\;L\;&=&\;\;1,000\;mL\\9\;L\;&=&\;9,000\;mL\end{eqnarray*}[/latex]
Step 2: Convert mL to cm3
[latex]\begin{eqnarray*}1\;mL\;&=&\;\;1\;cm^3\\&=&\;9000\;cm^3\end{eqnarray*}[/latex]
c. 35 cm3 = (?) cL
Step 1: Convert cm3 to mL.
[latex]\begin{eqnarray*}1\;cm^3\;&=&\;1\;mL\\35\;cm^3\;&=&\;35\;mL\end{eqnarray*}[/latex]
Step 2: Move 1 decimal place left.
[latex]= 3.5 cL[/latex]
d. 450 kg = (?) L
Step 1: Convert kg to g.
[latex]\begin{eqnarray*}1\;kg\;&=&\;1,000\;g\\450\;kg\;&=&\;450,000\;g\end{eqnarray*}[/latex]
Step 2: Convert g to mL.
[latex]\begin{eqnarray*}1\;g\;&=&\;1\;mL\\&=&\;450,000\;mL\end{eqnarray*}[/latex]
Step 3: Covert mL to L.
[latex]\begin{eqnarray*}1\;L\;&=&\;1,000\;mL\\&=&\;450\;L\end{eqnarray*}[/latex]
Example 2.1.14
A swimming pool measures 10 m by 8 m by 2 m. How many kilolitres of water will it hold?
Solution
Step 1: Find the volume in [latex]m^3[/latex].
[latex]160\;m^3\;=\;(\;?\;)\;kL[/latex]
[latex]V\;=\;w\times\;l\;\times\;h\;=\;(8m)\;(10m)\;(2m)\;=\;160\;m^3[/latex]
Step 2: Convert to cm3
[latex]1 m = 100 cm[/latex], [latex]3 × 2 = 6[/latex], move [latex]6[/latex] places right for volume.
[latex]160m^3\;=\;160,000,000\;cm^3[/latex]
Step 3: Convert to mL
[latex]\begin{eqnarray*}1\;mL\;&=&\;1\;cm^3\\160,000,000\;cm^3\;&=&\;160,000,000\;mL\end{eqnarray*}[/latex]
Step 4: Convert to kL.
[latex]\begin{eqnarray*}160,000,000\;mL\;&=&\;160\;kL\\1\;kL\;&=&\;1,000,000\;mL\\160\;m^3\;&=&\;160\;kL\\\end{eqnarray*}[/latex]
The swimming pool will hold 160 kL of water.
Use Mixed Units of Measurement in the Metric System
Performing arithmetic operations on measurements with mixed units of measures in the metric system requires care. Make sure to add or subtract like units.
Example 2.1.15
Ryland is 1.6 meters tall. His younger brother is 85 centimeters tall. How much taller is Ryland than his younger brother?
Solution
We can convert both measurements to either centimeters or meters. Since meters is the larger unit, we will subtract the lengths in meters. We convert 85 centimeters to meters by moving the decimal 2 places to the left.
Step 1: Write the 85 centimeters as meters.
85cm is 0.85m.
Step 2: Subtract.
[latex]\begin{align*} 1.6m&\\ \underline{-0.85m}&\\ 0.75m& \end{align*}[/latex]
Ryland is 0.75 m taller than his brother.
Try It
15) Mariella is 1.58 meters tall. Her daughter is 75 centimeters tall. How much taller is Mariella than her daughter? Write the answer in centimeters.
Solution
83 centimeters
16) The fence around Hank’s yard is 2 meters high. Hank is 96 centimeters tall. How much shorter than the fence is Hank? Write the answer in meters.
Solution
1.04 meters
Make Unit Conversions in the U.S. System
There are two systems of measurement commonly used around the world. Most countries use the metric system. The U.S. uses a different system of measurement, usually called the U.S. system. We will look at the U.S. system now.
The U.S. system of measurement uses units of inch, foot, yard, and mile to measure length and pound and ton to measure weight. For capacity, the units used are cup, pint, quart, and gallons. Both the U.S. system and the metric system measure time in seconds, minutes, and hours.
The equivalencies of measurements are following, also shows, in parentheses, the common abbreviations for each measurement.
| Length | 1 foot (ft.) = 12 inches (in.) | Volume | 3 teaspoons (t) = 1 tablespoon (T) |
| 1 yard (yd.) = 3 feet (ft.) | 16 tablespoons(T) = 1 cup (C) | ||
| 1 mile (mi.) = 5,280 feet (ft.) | 1 cup (C) = 8 fluid ounces (fl. oz.) | ||
| 1 pint (pt.) = 2 cups (C) | |||
| 1 quart (qt) = 2 pints (pt.) | |||
| 1 gallon (gal) = 4 quarts (qt.) | |||
| Weight | 1 pound (lb.) = 16 ounces (oz.) | Time | 1 minute = 60 seconds (sec) |
| 1 ton = 2205 pounds (lb.) | 1 hour (hr) = 60 minutes (min) | ||
| 1 day = 24 hours (hr) | |||
| 1 week (wk) = 7 days | |||
| 1 year (yr) = 365 days |
In many real-life applications, we need to convert between units of measurement, such as feet and yards, minutes and seconds, quarts and gallons, etc. We will use the identity property of multiplication to do these conversions. We’ll restate the identity property of multiplication here for easy reference.
Identity Property of Multiplication
For any real number [latex]a[/latex]: [latex]a\cdot 1=a[/latex] [latex]1\cdot a=a[/latex]
1 is the multiplicative identity
As we saw earlier in the section, dimensional analysis can be used to convert units. In the U.S System, since it is not a decimal system, it is best that we always use dimensional analysis to convert our units. Here, we elaborate on that concept.
To use the identity property of multiplication, we write 1 in a form that will help us convert the units. For example, suppose we want to change inches to feet. We know that 1 foot is equal to 12 inches, so we will write 1 as the fraction [latex]\frac{1 foot}{12 inch}[/latex] When we multiply by this fraction we do not change the value, but just change the units.
But [latex]\frac{1 foot}{12 inch}[/latex] also equals 1. How do we decide whether to multiply by [latex]\frac{1 foot}{12 inch}[/latex] or [latex]\frac{1 foot}{12 inch}[/latex]? We choose the fraction that will make the units we want to convert from divide out. Treat the unit words like factors and “divide out” common units like we do common factors. If we want to convert 66 inches to feet, which multiplication will eliminate the inches?
[latex]66\;inches\cdot\frac{1\;foot}{12\;inches}[/latex] or [latex]\xcancel{66\;inches\cdot\frac{12\;inches}{1\;foot}}[/latex]
The first form works since [latex]66\;\cancel{inches}\cdot\frac{1\;foot}{12\;\cancel{inches}}[/latex]
The inches divide out and leave only feet. The second form does not have any units that will divide out and so will not help us.
Example 2.1.16
Solution
Step 1: Multiply the measurement to be converted by 1; write 1 as a fraction relating the units given and the units needed.
Multiply 66 inches by 1, writing 1 as a fraction relating inches and feet. We need inches in the denominator so that the inches will divide out!
[latex]66\;\text{inches}\operatorname{×}1=66\;\text{inches }\times\frac{1\;\text{foot}}{12\;\text{inches}}\\[/latex]
Step 2: Multiply.
Think of 66 inches as [latex]\frac{66\;\text{inches}}1[/latex]
[latex]\frac{66\;\text{inches}\;\times1\;\text{foot}}{12\;\text{inches}}[/latex]
Step 3: Simplify the fraction.
Notice: inches divide out.
[latex]66\;\cancel{\text{inches}}\times\frac{1\text{ foot}}{12\;\cancel{\text{inches}}}=\frac{66\;\text{inches}}{12}[/latex]
Step 4: Simplify.
[latex]\text{Divide}\;66\;\text{by}\;12.[/latex]
[latex]5.5 feet[/latex]
Try It
17) Lexie is 30 inches tall. Convert her height to feet.
Solution
2.5 feet
18) Rene bought a hose that is 18 yards long. Convert the length to feet.
Solution
54 feet
How To
Make Unit Conversions.
- Multiply the measurement to be converted by 1; write 1 as a fraction relating the units given and the units needed.
- Multiply.
- Simplify the fraction.
- Simplify.
When we use the identity property of multiplication to convert units, we need to make sure the units we want to change from will divide out. Usually this means we want the conversion fraction to have those units in the denominator.
Example 2.1.17
Eli’s six months son is 102.4 ounces. Convert his weight to pounds.
Solution
To convert ounces into pounds we will multiply by conversion factors of 1.
Step 1: Write 1 as [latex]\frac{1\;\text{pound}}{16\;\text{ounces}}[/latex].
[latex]102.4\;\text{ounces}\times\frac{1\;\text{pound}}{16\;\text{ounces}}[/latex]
Step 2: Divide out the common units.
[latex]102.4\;\cancel{\text{ounces}}\times\frac{1\;\text{pound}}{16\;\cancel{\text{ounces}}}[/latex]
Step 3: Simplify the fraction.
[latex]\frac{102.4\;\text{ounces}}{16\;\text{ounces}}[/latex]
Step 4: Simplify.
[latex]6.4\;\text{pounds}[/latex]
Eli’s six months son weights 6.4 pounds.
Example 2.1.18
Ndula, an elephant at the San Diego Safari Park, weighs almost 3.2 tons. Convert her weight to pounds.
Solution
We will convert 3.2 tons into pounds. We will use the identity property of multiplication, writing 1 as the fraction:
[latex]\frac{2000\;\text{pounds}}{1\;\text{ton}}[/latex]
Step 1: Multiply the measurement to be converted, by 1.
[latex]3.2\;\text{tons}\times1[/latex]
Step 2: Write 1 as a fraction relating tons and pounds.
[latex]3.2\;\text{tons}\times\frac{2,000\;\text{pounds}}{1\;\text{ton}}[/latex]
Step 3: Simplify.
[latex]\frac{3.2\;\cancel{\text{tons}\;}\times\;2,000\;\text{pounds}}{1\;\cancel{\text{ton}}}[/latex]
Step 4: Multiply.
[latex]6,400\;\text{pounds}[/latex]
Try It
19) Arnold’s SUV weighs about 4.3 tons. Convert the weight to pounds.
Solution
8,600 pounds
20) The Carnival Destiny cruise ship weighs 51,000 tons. Convert the weight to pounds.
Solution
102,000,000 pounds
21) One year old girl weights 11 pounds. Convert her weight to ounces.
Solution
176 ounces.
As was the case with the metric system, sometimes, to convert from one unit to another, we may need to use several other units in between, so we will need to multiply several fractions.
Example 2.1.19
Juliet is going with her family to their summer home. She will be away from her boyfriend for 9 weeks. Convert the time to minutes.
Solution
To convert weeks into minutes we will convert weeks into days, days into hours, and then hours into minutes. To do this we will multiply by conversion factors of 1.
Step 1: Write 1 as [latex]\frac{7\;\text{days}}{1\;\text{week}}[/latex] , [latex]\frac{24\;\text{hours}}{1\;\text{day}}[/latex], and [latex]\frac{60\;\text{minutes}}{1\;\text{hour}}[/latex].
[latex]\frac{9 wk}{1}\times \frac{7 days}{1 wk}\times \frac{24 hr}{1 day}\times \frac{60 min}{1 hr}[/latex]
Step 2: Divide out the common units.
[latex]{\frac{9\;\cancel{wk}}1\cdot\frac{7\;}{\color{blue}{\cancel{days}}}{}{1\cancel{wk}}\cdot\frac{24\;{\color{red}{\cancel{hr}}}}{1\;{\color{blue}{\cancel{day}}}}\cdot\frac{60\;min}{1\;}{\color{red}{\cancel{hr}}}}[/latex]
Step 3: Multiply.
[latex]\frac{9\;\times\;7\;\times\;24\;\times\;60\;\text{min}}{1\;\times\;1\;\times\;1\;\times\;1}\;=90,720\;\text{minutes}[/latex]
Step 4: Multiply.
Juliet and her boyfriend will be apart for 90,720 minutes (although it may seem like an eternity!).
Try It
22) The distance between the earth and the moon is about 250,000 miles. Convert this length to yards.
Solution
440,000,000 yards
23) The astronauts of Expedition 28 on the International Space Station spend 15 weeks in space. Convert the time to minutes.
Solution
151,200 minutes
Example 2.1.20
How many ounces are in 1 gallon?
Solution
We will convert gallons to ounces by multiplying by several conversion factors. Refer to Table 2.1.5.
Step 1: Multiply the measurement to be converted by 1.
[latex]\frac{1\;\text{gallon}}1\times\frac{4\;\text{quarts}}{1\;\text{gallon}}\times\frac{2\;\text{pints}}{1\;\text{quart}}\times\frac{2\;\text{cups}}{1\;\text{pint}}\times\frac{8\;\text{ounces}}{1\;\text{cup}}[/latex]
Step 2: Use conversion factors to get to the right unit.
Simplify.
[latex]\frac{1\;\cancel{\text{gallon}}}1\times\frac{4\;\cancel{quarts}}{1\;\cancel{\text{gallon}}}\times\frac{2\;\cancel{\text{pints}}}{1\;\cancel{\text{quarts}}}\times\frac{2\;\cancel{\text{cups}}}{1\;\cancel{\text{pint}}}\times\frac{8\;\text{ounces}}{1\;\cancel{\text{cup}}}[/latex]
Step 3: Multiply.
[latex]\frac{1\times4\times2\times2\times8\;\text{ounces}}{1\times1\times1\times1\times1}[/latex]
Step 4: Simplify.
[latex]128\;\text{ounces}[/latex]
There are 128 ounces in a gallon.
Try It
24) How many cups are in 1 gallon?
Solution
16 cups
25) How many teaspoons are in 1 cup?
Solution
48 teaspoons
Use Mixed Units of Measurement in the U.S. System
We often use mixed units of measurement in everyday situations. Suppose Joe is 5 feet 10 inches tall, stays at work for 7 hours and 45 minutes, and then eats a 1 pound 2 ounce steak for dinner—all these measurements have mixed units.
Performing arithmetic operations on measurements with mixed units of measures requires care. Be sure to add or subtract like units!
Example 2.1.21
Seymour bought three steaks for a barbecue. Their weights were 14 ounces, 1 pound 2 ounces and 1 pound 6 ounces. How many total pounds of steak did he buy?
Solution
We will add the weights of the steaks to find the total weight of the steaks.
Step 1: Add the ounces. Then add the pounds.
[latex]\begin{eqnarray*}&+&\;14\;\text{ounces}\\1\;\text{pound}\;&+&\;2\;\text{ounces}\\1\;\text{pound}\;&+&\;6\;\text{ounces}\\=\;2\;\text{pounds}\;&+&\;22\;\text{ounces}\end{eqnarray*}[/latex]
Step 2: Convert 22 ounces to pounds and ounces.
2 pounds 1 pound, 6 ounces.
Step 3: Add the pounds.
3 pounds, 6 ounces.
Seymour bought 3 pounds 6 ounces of steak.
Try It
26) Laura gave birth to triplets weighing 3 pounds 3 ounces, 3 pounds 3 ounces, and 2 pounds 9 ounces. What was the total birth weight of the three babies?
Solution
9 lbs. 8 oz
27) Stan cut two pieces of crown moulding for his family room that were 8 feet 7 inches and 12 feet 11 inches. What was the total length of the moulding?
Solution
21 ft. 6 in.
Example 2.1.22
Anthony bought four planks of wood that were each 6 feet 4 inches long. What is the total length of the wood he purchased?
Solution
We will multiply the length of one plank to find the total length.
Step 1: Multiply the inches and then the feet.
[latex]\begin{eqnarray*} \;\;6\;feet\;\;\;4\;inches\\ \underline{\times\phantom{\rule{6em}{0ex}}4\phantom{\rule{3em}{0ex}}}\\ 24\;feet\;\;16\;inches \end{eqnarray*}[/latex]
Step 2: Convert the 16 inches to feet.
[latex]{\begin{eqnarray*}16\;\text{inches}\;-\;12\;\text{inches}\;&=&\;{\color{red}{1\;\text{foot}\;+\;4\;\text{inches}}}\\{\color{red}{1\;\text{foot}\;+\;4\;\text{inches}\;+\;24\;\text{feet}}}\;&=&\;25\;\text{feet}\;+\;4\;\text{inches}\end{eqnarray*}}[/latex]
Step 3: Add the feet.
Anthony bought 25 feet and 4 inches of wood.
Try It
28) Henri wants to triple his spaghetti sauce recipe that uses 1 pound 8 ounces of ground turkey. How many pounds of ground turkey will he need?
Solution
4 lbs. 8 oz.
29) Joellen wants to double a solution of 5 gallons 3 quarts. How many gallons of solution will she have in all?
Solution
11 gallons 2 qt.
Convert Between the U.S. and the Metric Systems of Measurement
Many measurements in the United States are made in metric units. The soda may come in 2-liter bottles, calcium may come in 500-mg capsules, and people may run a 5K race. To work easily in both systems, we need to be able to convert between the two systems.
The table below shows some of the most common conversions.
Conversion Factors Between U.S. and Metric Systems
| Length | Mass | Capacity |
|---|---|---|
| 1 in. = 2.54 cm | 1 lb. = 0.45 kg | 1 qt. = 0.95 L |
| 1 ft. = 0.305 m | 1 oz. = 28 g | 1 fl. oz. = 30 mL |
| 1 yd. = 0.914 m | 1 kg = 2.2 lb. | 1 L = 1.06 qt. |
| 1 mi. = 1.61 km | ||
| 1 m = 3.28 ft. |
Figure 2.1.2 shows how inches and centimeters are related on a ruler.
Figure 2.1.3 shows the ounce and milliliter markings on a measuring cup.
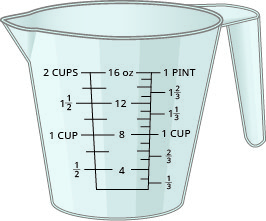
Figure 2.1.4 shows how pounds and kilograms marked on a bathroom scale.
We make conversions between the systems just as we do within the systems—by multiplying by unit conversion factors.
Example 2.1.23
Solution
Step 1: Multiply by a unit conversion factor relating mL and ounces.
[latex]500\;\text{millilitres}\cdot\frac{1\;\text{ounce}}{30\;\text{millilitres}}[/latex]
Step 2: Simplify.
[latex]\frac{50\;\text{ounces}}{30}[/latex]
Step 3: Divide.
[latex]16.7\;\text{ounces}[/latex]
The plastic bag has 16.7 ounces of packed red cells.
Try It
30) Adam donated 450 ml of blood. How many ounces is that?
Solution
15 ounces.
31) How many quarts of soda are in a 2-L bottle?
Solution
2.12 quarts
32)How many liters are in 4 quarts of milk?
Solution
3.8 liters
Example 2.1.24
Soleil was on a road trip and saw a sign that said the next rest stop was in 100 kilometers. How many miles until the next rest stop?
Solution
Step 1: Multiply by a unit conversion factor relating km and mi.
[latex]100\;\text{kilometers}\cdot\frac{1\;\text{mile}}{1.61\;\text{kilometer}}[/latex]
Step 2: Simplify.
[latex]\frac{100\;\text{miles}}{1.61}[/latex]
Step 3: Divide.
[latex]62\;\text{miles}[/latex]
Soleil will travel 62 miles.
Example 2.1.25
A human brain weights about 3 pounds. How many kilograms is that? Round to the nearest tenth of a kilogram.
Solution
Step 1: Multiply by a unit conversion factor relating km and mi.
[latex]3\;\text{pounds}\times\frac{1\;\text{kilogram}}{2.2\;\text{pounds}}[/latex]
Step 2: Simplify.
[latex]\frac{3\;\text{kilograms}}{2.2}[/latex]
Step 3: Divide.
[latex]1.4\;\text{kilograms}[/latex]
A human brain weights around 1.4 kilograms.
Try It
33) A human liver normally weights approximately 1.5 kilograms. Convert it to pounds.
Solution
3.3 pounds
34) The height of Mount Kilimanjaro is 5,895 meters. Convert the height to feet.
Solution
19,335.6 feet
35) The flight distance from New York City to London is 5,586 kilometers. Convert the distance to miles.
Solution
8,993.46 km
Convert between Fahrenheit and Celsius Temperatures
Have you ever been in a foreign country and heard the weather forecast? If the forecast is for 79°F what does that mean?
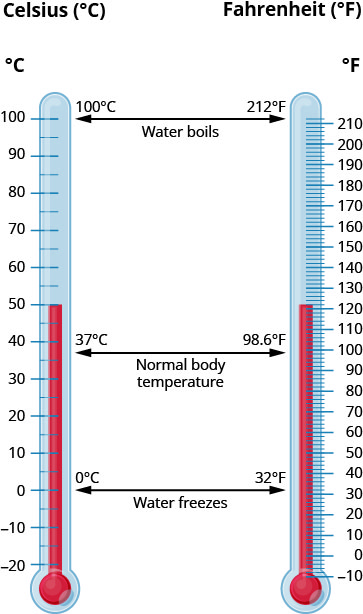
The U.S. and metric systems use different scales to measure temperature. The U.S. system uses degrees Fahrenheit, written °F The metric system uses degrees Celsius, written °C. Figure 2.1.5 shows the relationship between the two systems. The diagram shows normal body temperature, along with the freezing and boiling temperatures of water in degrees Fahrenheit and degrees Celsius.
Temperature Conversion
To convert from Fahrenheit temperature, F, to Celsius temperature, C, use the formula:
[latex]C=\frac{5}{9}(F-32)[/latex]
To convert from Celsius temperature, C, to Fahrenheit temperature, F, use the formula:
[latex]F=\frac{9}{5}C+32[/latex]
Example 2.1.26
Convert 50° Fahrenheit into degrees Celsius.
Solution
We will substitute 50°F into the formula to find C.
Step 1: Substitute 50 for F.
[latex]{C=\frac59\left({\color{red}{50}}-32\right)}[/latex]
Step 2: Simplify in parentheses.
[latex]C=\frac59\left(18\right)[/latex]
Step 3: Multiply.
[latex]C=10[/latex]
So we found that 50°F is equivalent to 10°C.
Example 2.1.27
Before mixing, the Pfizer-BioNTech COVID-19 vaccine may be stored in an ultra-cold freezer between -112°F and -76°F. Convert the temperatures into degrees Celsius.
Solution
We will substitute a) -112°F and b) -76°F into the formula to find C.
a.
Step 1: Substitute -112 for F.
[latex]C=\frac{5}{9}(-112-32)[/latex]
Step 2: Simplify in parentheses.
[latex]C=\frac{5}{9}(-144)[/latex]
Step 3: Multiply.
[latex]C= - 80[/latex]
So we found that -112°F is equivalent to -80°C
b.
Step 1: Substitute -76 for F.
[latex]C=\frac59(-76-32)[/latex]
Step 2: Simplify.
[latex]\begin{eqnarray*}C&=&\frac{5}{9}(-108)\\C&=&-60\end{eqnarray*}[/latex]
So we found that -76°F is equivalent to – 60°C.
Try It
36) Convert the Fahrenheit temperature to degrees Celsius: 59° Fahrenheit.
Solution
15°C
37) Convert the Fahrenheit temperature to degrees Celsius: 41° Fahrenheit.
Solution
5°C
Example 2.1.28
While visiting Paris, Woody saw the temperature was 20° Celsius. Convert the temperature into degrees Fahrenheit.
Solution
We will substitute 20°C into the formula to find F.
Step 1: Substitute 20 for C.
[latex]{F=\frac95\left({\color{red}{20}}\right) 32}[/latex]
Step 2: Multiply.
[latex]F=36 32[/latex]
Step 3: Add.
[latex]F=68[/latex]
So we found that 20°C is equivalent to 68°F.
Example 2.1.29
Once mixed, the Pfizer-BioNTech COVID-19 vaccine can be left at room temperature 2°C to 25°C. Convert the temperatures into degrees Fahrenheit.
Solution
We will substitute a) 2°C and b) 25°C into the formula to find F.
a.
Step 1: Substitute 2 for C.
[latex]F=\frac{9}{5}\times \text{2} 32[/latex]
Step 2: Simplify.
[latex]F= 35.6[/latex]
So we found that 2°C is equivalent to 35.6°F.
b.
Step 1: Substitute 25 for C.
[latex]F=\frac{9}{5}\times\text{25} 32[/latex]
Step 2: Simplify.
[latex]F= 77[/latex]
So we found that 25°C is equivalent to 77°F.
Try It
38) Convert the Celsius temperature to degrees Fahrenheit: the temperature in Helsinki, Finland, was 15° Celsius.
Solution
59°F
39) Convert the Celsius temperature to degrees Fahrenheit: the temperature in Sydney, Australia, was 10° Celsius.
Solution
50°F
Key Concepts
- Metric System of Measurement
| Length | Mass | Capacity |
|---|---|---|
| 1 kilometer (km) = 1,000 m 1 hectometer (hm) = 100 m 1 dekameter (dam) = 10 m 1 meter (m) = 1 m 1 decimeter (dm) = 0.1 m 1 centimeter (cm) = 0.01 m 1 millimeter (mm) = 0.001 m |
1 kilogram (kg) = 1,000 g 1 hectogram (hg) = 100 g 1 dekagram (dag) = 10 g 1 gram (g) = 1 g 1 decigram (dg) = 0.1 g 1 centigram (cg) = 0.01 g 1 milligram (mg) = 0.001 g |
1 kiloliter (kL) = 1,000 L 1 hectoliter (hL) = 100 L 1 dekaliter (daL) = 10 L 1 liter (L) = 1 L 1 deciliter (dL) = 0.1 L 1 centiliter (cL) = 0.01 L 1 milliliter (mL) = 0.001 L |
| 1 meter = 100 centimeters 1 meter = 1,000 millimeters |
1 gram = 100 centigrams 1 gram = 1,000 milligrams |
1 liter = 100 centiliters 1 liter = 1,000 milliliters |
- Temperature Conversion
- To convert from Fahrenheit temperature, F, to Celsius temperature, C, use the formula [latex]C=\frac{5}{9}(F-32)[/latex]
- To convert from Celsius temperature, C, to Fahrenheit temperature, F, use the formula [latex]F=\frac{9}{5}C+32[/latex]
Self Check
After completing the exercises, use this checklist to evaluate your mastery of the objectives of this section.
Overall, after looking at the checklist, do you think you are well-prepared for the next Chapter? Why or why not?