Absorption spectrometry: using measurements at different light frequencies
The Beer-Lambert law postulates that the logarithm of the ratio of the light intensities is a linear function of the concentrations of each gas in the mix. The log-ratio of intensities is thus of the form ![]() for some vector
for some vector ![]() , where
, where ![]() is the vector of concentrations, and the vector
is the vector of concentrations, and the vector ![]() contains the coefficients of absorption of each gas. This vector is actually also a function of the frequency of the light we illuminate the container with.
contains the coefficients of absorption of each gas. This vector is actually also a function of the frequency of the light we illuminate the container with.
Now consider a container having a mixture of ![]() ‘‘pure’’ gases in it. Denote by
‘‘pure’’ gases in it. Denote by ![]() the vector of concentrations of the gases in the mixture. We illuminate the container at different frequencies
the vector of concentrations of the gases in the mixture. We illuminate the container at different frequencies ![]() . For each experiment, we record the corresponding log-ratio
. For each experiment, we record the corresponding log-ratio ![]() , of the intensities. If the Beer-Lambert law is to be believed, then we must have
, of the intensities. If the Beer-Lambert law is to be believed, then we must have
![]()
for some vectors ![]() , which contain the coefficients of absorption of the gases at light frequency
, which contain the coefficients of absorption of the gases at light frequency ![]() . More compactly:
. More compactly:
![]()
where
![Rendered by QuickLaTeX.com \[A = \begin{pmatrix} a_{1}^{T} \\ \vdots \\ a_{m}^{T} \end{pmatrix}.\]](https://ecampusontario.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-116a7662dec8008d9855e30f542fb5ae_l3.png)
Thus, ![]() is the coefficient of absorption of the
is the coefficient of absorption of the 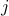 -th gas at frequency
-th gas at frequency ![]() .
.
Since ![]() ‘s correspond to ‘‘pure’’ gases, they can be measured in the laboratory. We can then use the above model to infer the concentration of the gases in a mixture, given some observed light intensity log-ratio.
‘s correspond to ‘‘pure’’ gases, they can be measured in the laboratory. We can then use the above model to infer the concentration of the gases in a mixture, given some observed light intensity log-ratio.
