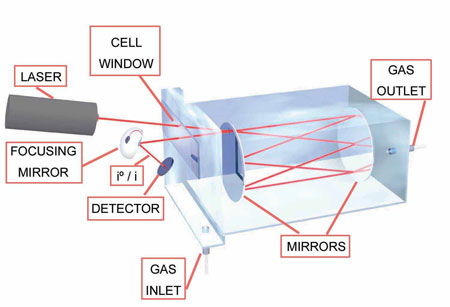
Beer-Lambert Law in Absorption Spectrometry
The Beer-Lambert law in optics is an empirical relationship that relates the absorption of light by a material, to the properties of the material through which the light is traveling. This is the basis of absorption spectrometry, which allows to measure the concentration of different gases in a chamber.
If the container has a mixture of ![]() ‘‘pure’’ gases in it, the law postulates that the logarithm of the ratio of the light intensities is a linear function of the concentrations of each gas in the mix. The log-ratio of intensities is thus of the form
‘‘pure’’ gases in it, the law postulates that the logarithm of the ratio of the light intensities is a linear function of the concentrations of each gas in the mix. The log-ratio of intensities is thus of the form ![]() for some vector
for some vector ![]() , where
, where ![]() is the vector of concentrations. The coefficients
is the vector of concentrations. The coefficients ![]() ,
, ![]() correspond to the log-ratio of light intensities when
correspond to the log-ratio of light intensities when ![]() (the
(the ![]() -th vector of the standard basis, which correspond to the
-th vector of the standard basis, which correspond to the ![]() -th pure gas). The quantity
-th pure gas). The quantity ![]() is called the coefficient of absorption of the
is called the coefficient of absorption of the ![]() -th gas and can be measured in the laboratory.
-th gas and can be measured in the laboratory.
See also: Absorption spectrometry: using measurements at different light frequencies.