8.2 Arterial Blood Gases
Now that we reviewed the basics of acid-base balance and pH in the body, let’s talk a little more about Arterial Blood Gases (ABGs). Arterial blood is being pumped from the heart after getting oxygenated by the lungs, and is being delivered to the organs. This blood has not dropped off the oxygen at all the cells yet and therefore, the levels of CO2 and O2 are reflective of how well the lungs have been able to clear the CO2 and add oxygen after going through the lungs.
ABGs convey four different values (often Base Excess is added as the fifth value):
- pH
- pCO2
- pO2
- HCO3
You will notice there is a “p” in front of the CO2 and O2 in an ABG. This is because the levels of CO2 and O2 are expressed as a partial pressure. Don’t get caught up in that. It is just a way of giving the levels units that the numbers can be expressed in. It is still reflective of the levels of CO2 and O2 in the blood, and we will go through how to read them.
Normal Values
Take a look at this brief table which presents the normal ranges for all of the values in the ABG results. When results are outside of these ranges, they tell you that something needs to be done for your patient. Each of these normal ranges and what they mean is discussed in more detail below.
|
Normal Values |
|
|---|---|
| [latex]\text{pH}[/latex] | [latex]7.35\text{ - }7.45[/latex] |
| [latex]\text{pCO}_2[/latex] | [latex]\text{35 - 45 mmHg}[/latex] |
| [latex]\text{pO}_2[/latex] | [latex]\text{80 - 100 mmHg}[/latex] |
| [latex]\text{HCO}_3[/latex] | [latex]\text{22 - 28 mmol/L}[/latex] |
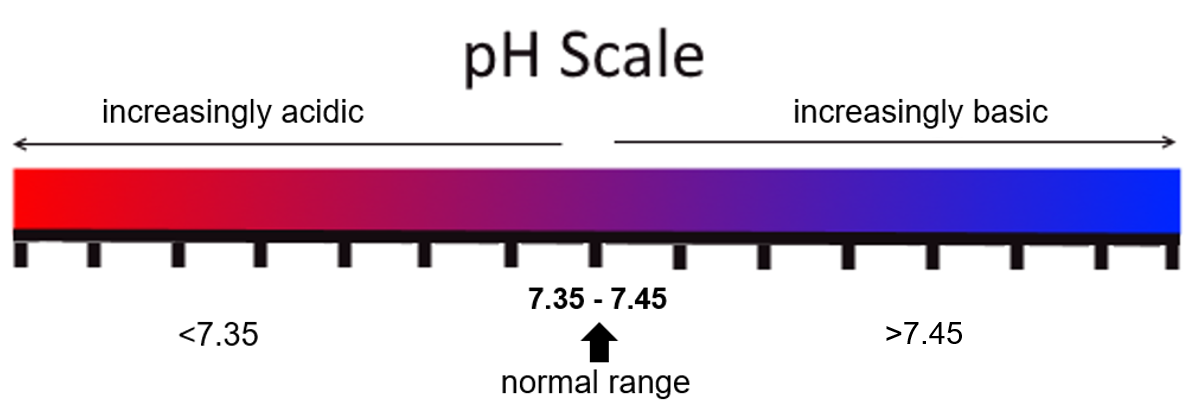
As previously discussed, the pH of the blood should be between [latex]7.35\text{ - }7.45[/latex] to be considered within normal range. If the pH is below [latex]7.35[/latex], it is considered acidic; and if the pH is above [latex]7.45[/latex], it is considered alkaline.
Revisit the visual pH scale now, but notice that the numerical ranges have been added:
The partial pressure of CO2 in the blood is considered normal at [latex]\text{35 - 45 mmHg}[/latex]. If the CO2 is higher than [latex]45\text{ mmHg}[/latex], this is termed hypercarbia (or hypercapnia). If the CO2 is less than [latex]35\text{ mmHg}[/latex] this is hypocarbia (hypocapnia). Another term that is used to discuss this type of reading is CO2 retention.
Key Takeaway
Remember! CO2 = acid; therefore, low CO2 = alkalosis (alkaline pH), and high CO2 = acidosis (acidic pH).
The partial pressure of oxygen in the blood should be [latex]\text{80 - 100 mmHg}[/latex] when at normal levels. This number is read off the blood gas, but is separate from the acid-base process. The pO2 reading is not part of your determination of acid-base and is generally looked at after your ABG interpretation to assess the oxygenation status. You will learn more about pO2 later in the chapter after ABG interpretations (see Oxygenation Status: The Final Piece).
The concentration of HCO3 in the blood is somewhere around [latex]\text{22 - 28 mmols/L}[/latex] (each textbook/hospital may vary slightly in their normal range—for the purpose of this book, this will be our normal range). Bicarbonate levels higher than 28 will shift the body into a state of alkalosis. Low bicarbonate levels less than 22 will not be able to buffer free H+ ions, allowing the body to go into a state of acidosis.
Key Takeaway
Remember! HCO3 = base; therefore, low HCO3 = acidosis and high HCO3 = alkalosis.
When interpreting an ABG, the values are normally reported in this order:
- pH,
- pCO2,
- PO2 and then
- HCO3.
ABG shorthand is often written with the numbers in that order with backslashes between them (pH/pCO2/pO2/HCO3). Don’t let this confuse you. Just remember the order and you will look like a pro!
Let’s uncomplicate this. Before jumping into how to start interpreting blood gases, we are going to go through an analogy to help you understand the science behind what is going on when it comes to ABGs. Truly understanding the concept is always better than trying to memorize patterns. Take a look at the following object lesson.
Object Lesson

Think of homeostasis as a massive game of Tug O’ War. The CO2 family (Team Acid) and the Bicarbonate Family (Team Base) are locked in an epic battle that never seems to end. Normally, the CO2 family has 35-45 members (normal pCO2 levels) while the Bicarbonate Family has 22-28 members (normal HCO3 levels in the body). At these levels, the teams are evenly matched and no one is winning. At this balanced or “normal” level, the pH in the body is [latex]7.35\text{ - }7.45[/latex].
If Team Acid is winning, they have been able to pull the rope to their side, pulling the pH rope past the 7.35 mark—any pH under 7.35 is classified as an acid. If Team Base is winning, they have been able to pull the pH rope past the 7.45 mark—any pH over 7.45 is considered base. If the game of Tug O’ War is perfectly matched with 35-45 pCO2 on one side and 22-28 HCO3 on the other side, changes in the number of players on either side will have direct impact on which team is winning.
If the CO2 family calls their friends in to join the team (more CO2) the pH/rope is going to start to move toward the acid side. Conversely, if the CO2 side is at their normal player numbers, but the bicarbonate family calls for extra teammates to come join, the pH/rope will pull towards the base side. It is also very important to realize that if both teams are at their normal amounts, any loss of players on either side will cause the opposite side to start to pull the pH/rope their way. If the HCO3 family loses some of their teammates, then the rope starts to go more toward Team Acid, or if the CO2 family loses members, then Team Base will start to win the battle of the pH and pull the rope above 7.45 pH)
Here is the most important fact to remember: given the opportunity, the human body always wants to return to homeostasis (pH [latex]7.35\text{ - }7.45[/latex]). As pCO2 and HCO3 start to change, the human body will start to compensate by altering the opposite side to try to even out the imbalance. Think of this as the CO2 and HCO3 family wanting a fair fight. So as teammates are lost on one side, then the other side will drop teammates as well because they want to beat the other team “fair and square.” Also, as one side adds players, the other side will also add players, if given the chance, to compensate and even out the game.
Keep this Tug O’ War analogy in mind when interpreting ABGs, and you will never go wrong.
"Arterial Blood Gases" from Basic Principles of Mechanical Ventilation by Melody Bishop, © Sault College is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License, except where otherwise noted.

