6.2 Earth
Earth has vast oceans of liquid water, large masses of exposed land, and a dynamic atmosphere with clouds of water vapour. Earth also has ice covering its polar regions. Earth’s average surface temperature is 14 degrees Celsius (57 degrees Fahrenheit). Water is a liquid at this temperature, but the planet also has water in its other two states, solid and gas. The oceans and the atmosphere help keep Earth’s surface temperatures reasonably steady.
Cross-Section of Earth
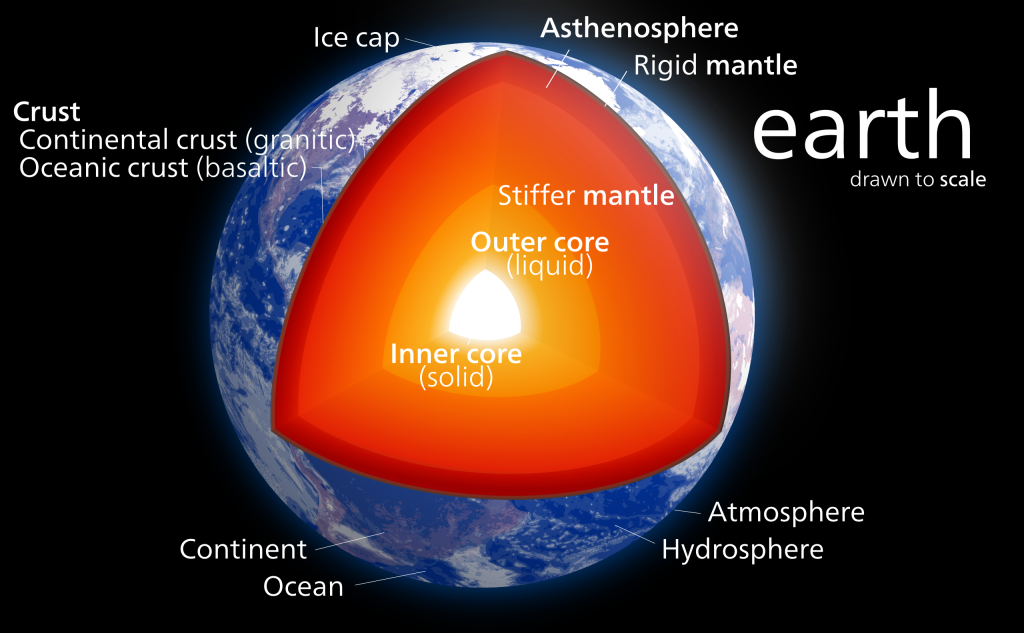
Earth by Kelvinsong, CC BY-SA 3.0.
Earth is the only planet known to have known life. The presence of liquid water, the atmosphere’s ability to filter out harmful radiation, and many other features make the planet uniquely suited to harbour life. Life and Earth now affect each other; for example, the evolution of plants allowed oxygen to enter the atmosphere in large enough quantities for animals to evolve. Although life has not been found elsewhere in the solar system, other planets or satellites may harbour primitive life forms. Life may also be found elsewhere in the universe.
The heat that remained from the planet’s accretion, gravitational compression, and radioactive decay allowed the Earth to melt, probably more than once. As it subsequently cooled, gravity pulled metal into the centre to create the core. Heavier rocks formed the mantle, and lighter rocks formed the crust.
Earth’s crust is divided into tectonic plates, which move around on the surface because of the convecting mantle below. The plates’ movement causes other geological activity, such as earthquakes, volcanoes, and mountains. The locations of these features are mostly related to current or former plate boundaries. Earth is the only planet known to have plate tectonics.
Earth rotates on its axis once per day, by definition. Earth orbits the Sun once every 365.24 days, which is defined as a year. Earth has one large moon, which orbits Earth once every 29.5 days, a period known as a month.
We live at the bottom of the ocean of air that envelops our planet. The atmosphere, weighing down upon Earth’s surface under the force of gravity, exerts a pressure at sea level that scientists define as 1 bar (a term that comes from the same root as barometer, an instrument used to measure atmospheric pressure). A bar of pressure means that each square centimetre of Earth’s surface has a weight equivalent to 1.03 kilograms pressing down on it. Humans have evolved to live at this pressure; make the pressure a lot lower or higher and we do not function well.
The total mass of Earth’s atmosphere is about 5 × 1018 kilograms. This sounds like a large number, but it is only about a millionth of the total mass of Earth. The atmosphere represents a smaller fraction of Earth than the fraction of your mass represented by the hair on your head.
The structure of the atmosphere is illustrated in the Figure 6.2. Most of the atmosphere is concentrated near the surface of Earth, within about the bottom 10 kilometres where clouds form and airplanes fly. Within this region—called the troposphere—warm air, heated by the surface, rises and is replaced by descending currents of cooler air; this is an example of convection. This circulation generates clouds and wind. Within the troposphere, temperature decreases rapidly with increasing elevation to values near 50 °C below freezing at its upper boundary, where the stratosphere begins. Most of the stratosphere, which extends to about 50 kilometres above the surface, is cold and free of clouds.
Structure of Earth’s Atmosphere
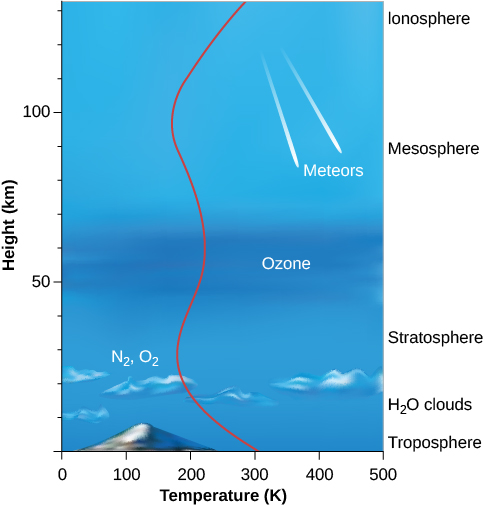
Near the top of the stratosphere is a layer of ozone (O3), a heavy form of oxygen with three atoms per molecule instead of the usual two. Because ozone is a good absorber of ultraviolet light, it protects the surface from some of the Sun’s dangerous ultraviolet radiation, making it possible for life to exist on Earth. The breakup of ozone adds heat to the stratosphere, reversing the decreasing temperature trend in the troposphere. Because ozone is essential to our survival, we reacted with justifiable concern to evidence that became clear in the 1980s that atmospheric ozone was being destroyed by human activities. By international agreement, the production of industrial chemicals that cause ozone depletion, called chlorofluorocarbons, or CFCs, has been phased out. As a result, ozone loss has stopped and the “ozone hole” over the Antarctic is shrinking gradually. This is an example of how concerted international action can help maintain the habitability of Earth.
At heights above 100 kilometres, the atmosphere is so thin that orbiting satellites can pass through it with very little friction. Many of the atoms are ionized by the loss of an electron, and this region is often called the ionosphere. At these elevations, individual atoms can occasionally escape completely from the gravitational field of Earth. There is a continuous, slow leaking of atmosphere—especially of lightweight atoms, which move faster than heavy ones. Earth’s atmosphere cannot, for example, hold on for long to hydrogen or helium, which escape into space. Earth is not the only planet to experience atmosphere leakage. Atmospheric leakage also created Mars’ thin atmosphere. Venus’ dry atmosphere evolved because its proximity to the Sun vaporized and dissociated any water, with the component gases lost to space.
Attribution
“2.5 Terrestrial Planets” from Physical Geography and Natural Disasters by R. Adam Dastrup, MA, GISP is licensed under a Creative Commons Attribution Non-Commerical Share-Alike 4.0 International License, except where otherwise noted.
“8.3 Earth’s Atmosphere” from Douglas College Astronomy 1105 by Douglas College Department of Physics and Astronomy, is licensed under a Creative Commons Attribution 4.0 International License, except where otherwise noted. Adapted from Astronomy 2e.

