2 Amino Acid Properties: Polarity and Ionization
Synopsis: Amino acid side chains may be classed according to polarity, hydrogen bonding ability and ionic properties. Biochemical reactions occur in aqueous solution at close to neutral pH. Many biochemical substances such as amino acids include weak acid groups such as carboxylates, SH or phenolic OH; or weak bases such as amines or some ring N compounds. The behaviour of such groups is highly dependent on whether they are protonated or deprotonated.
REVIEW: CHEM*1040 notes regarding weak acids and bases.
Amino acids with very non-polar side chains: Ala, Val, Leu, Ile, Phe, Met
These side chains are dominated by hydrocarbon, i.e. consists only of C-C and C-H bonds, e.g. Valine
Hydrocarbon is non-polar and hydrophobic, or water avoiding.
Polar and non-polar properties
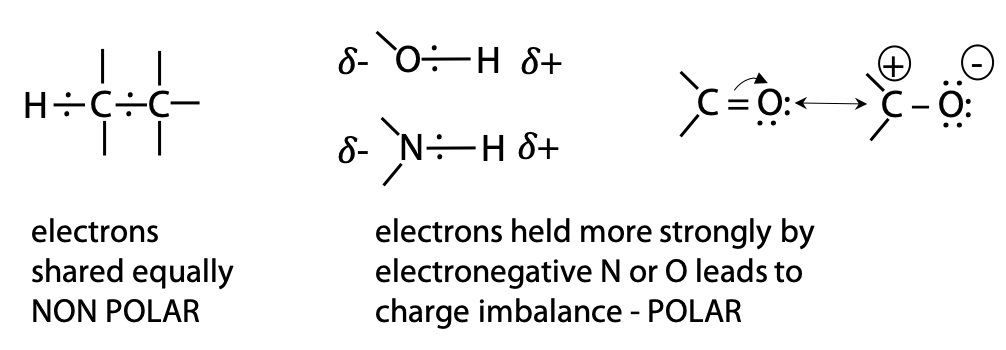
Polarity is a property that occurs when atoms in a molecule have very different electronegativity. Electronegativity refers to the tendency of a nucleus to hold electrons (it does NOT mean possession of negative charge).
very electronegative O > N > S > C ≈ H moderately electronegative
Since C and H have similar electronegativity, bonding electrons are evenly distributed in hydrocarbon regions. In contrast, O and N are more electronegative. In bonds such as O-H or C=O, electrons shift towards the electronegative O atom, creating a dipole (Figure 2.2). Therefore, we say that C=O and O-H are polar, while C-C and C-H are non-polar.
Non-polar groups interact well with each other and poorly with polar groups. Hydrocarbon regions of a molecule are also generally chemically unreactive.
Hydrocarbon side chains are described as hydrophobic, i.e. they avoid H2O, which is very polar. Hydrocarbon side chains tend to cluster together, so as to minimize the area of direct contact between hydrocarbon and H2O, a property known as the hydrophobic effect. The hydrophobicity of a side chain is simply related to the number of CH, CH2 or CH3 groups. Note: methionine has one S atom in its hydrocarbon chain. However, S is much less electronegative than O, so methionine fits in the very non-polar class.
Amino acids with moderately non polar side chains: Gly, Cys, Pro, Trp, Tyr
Glycine has a side chain that is simply H linked directly to the α-carbon: +NH3CH2CO2–
Although it’s a non-polar bond, it’s not large enough to make the whole molecule very non-polar.
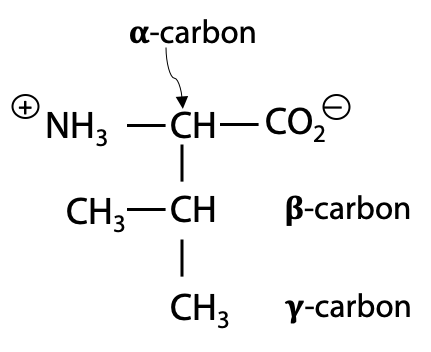
Note: The α-carbon of all other natural amino acids is chiral, with L- configuration.
Glycine is the only non-chiral structure, since it has two identical H substituents on the α-carbon.
Cysteine has the side chain -CH2-SH, which is not very polar, because S is much less electronegative than O.
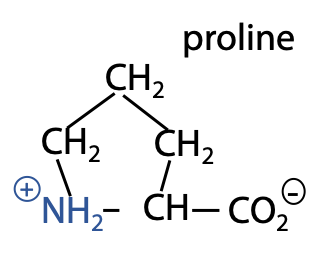
Proline is unique because the side chain links back to the -N, forming a 5-member ring (Figure 2.3).
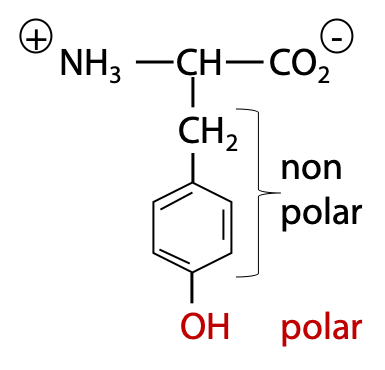
Tyrosine and tryptophan are the most hydrophobic amino acids, based on their total surface area of CH atoms. However, the hydrophobicity is partly offset by the presence of a polar OH group in Tyr (Figure 2.4), or slightly polar NH group in Trp. Therefore, in overall, these two amino acids are only somewhat non-polar.
Amino acids with polar, uncharged side chain: Ser, Thr, Asn, Gln
Ser and Thr both have an -OH group on the side chain.
Asn and Gln both have amide side chains -CONH2 derived from the corresponding carboxylates Asp and Glu.
All four are good hydrogen bond formers, with little hydrocarbon. Hence, they are polar.
Hydrogen bonds are electrostatic interactions between a donor consisting of the dipole of a polar O-H or N-H bond and an acceptor, consisting of an available lone pair of electrons on a nearby N or O atom (which may be on different molecule) (Figure 2.5).
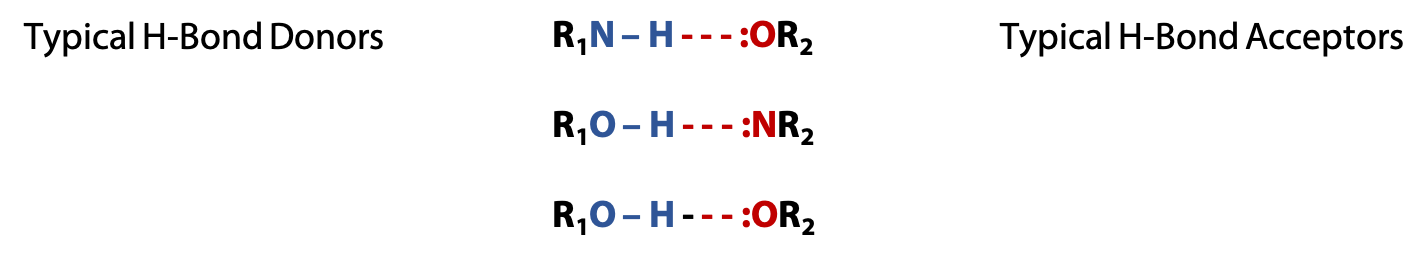
Typical hydrogen bonds (or H-bonds) are about 5-10% as strong as a normal covalent bond, but they are not permanent bonds like covalent bonds. Instead, they result in temporary attractive forces that help hold molecules together. Water molecules are excellent H-bond donors and acceptors. Therefore, polar amino acids interact well with H2O. H-bonding attracts H2O molecules to each other. This makes water a liquid rather than a gas like methane, CH4, whose molecules do not form H-bonds.
Biochemistry is concerned with how molecules function in living cells, which is an aqueous environment. Hence, it is important to understand how various biochemical molecules will interact with H2O.
Positively charged amino acids: Arg, Lys, His
These side chains are weak bases, fully protonated (Lys, Arg) or partly protonated (His) in normal biological conditions, pH 7.0-7.4.
Although the side chain has a hydrocarbon segment, the positive charge dominates over any hydrophobic effect. Charged amino acids are very polar.
e.g., The Lysine side chain is -CH2-CH2-CH2-CH2-NH3+
Negatively charged amino acids: Asp, Glu
These side chains are carboxylate groups, normally deprotonated at pH 7, and very polar.
The Aspartate side chain is -CH2-COO–.
The Glutamate side chain is -CH2-CH2-COO–.
Two amino acids with oppositely charged side chains can strongly attract each other by electrostatic interactions known as salt bridges or ion pairs (discussed in Chapter 7). They also form strong H-bonds with uncharged H-bond donors or acceptors including H2O.
Amino acids as weak electrolytes
Normal biochemical processes occur in aqueous solution close to neutral pH. Typical physiological pH is about 7.2 to 7.4, pH 7.0 is a close approximation. Certain functional groups found in biological molecules, in particular carboxylic acids or amino groups, can gain or lose H+ depending on the availability of hydrogen ions (or protons) in the solution.
pH expresses the availability of H+; pH = – log10 [H+]
Each ionic functional group, e.g. amino groups or carboxylic acid groups, has a characteristic constant, pKa, which expresses the tendency to gain or lose H+.
The Henderson Hasselbalch equation relates pH to pKa and the state of ionization:
(1) ![]()
The Henderson-Hasselbalch equation allows one to do the calculations needed:
- To determine the pH given by the ionic conditions of the surroundings. If pKa and the concentrations are known, pH can be calculated.
- To determine the degree of protonation or deprotonation of an ionizable functional group at a given pH. If the pH and pKa are known, the ratio of concentrations can be calculated. This means we can work out what the state of an “ionic” functional group actually is at a given pH.
For each amino acid, there is
- an α-carboxylic acid (typical pKa 2.4 ± 0.5)
- an α-amino group (typical pKa 9.6 ± 0.5)
TIPS:
- certain amino acids also have a side chain which may be charged
- Not all amino acids have a side chain that bears a charge!!
Exact pKa values for each of the 20 amino acids can be found in table 3.4 in the Stryer textbook (click here for the link).
NOTE: The pKa values given in Stryer and used here (see Table 2.1 below) vary slightly. You do not have to memorize these values. They will ALWAYS be given to you on tests.
The Henderson-Hasselbalch equation is used to calculate the state of each group at pH 7.0
(2) ![]()
![]()
Given pKa = 2.4 for the α-carboxylic acid group, we can calculate the ratio of anionic carboxylate -COO– to neutral carboxylic acid -COOH when pH = 7.0:
![]()
This indicates that the vast majority of α-carboxylic acid groups are fully deprotonated (i.e. have lost H+) and exist in the carboxylate ion state at pH 7.0.
Given pKa = 9.6 for the α-amino group,
![]()
This indicates that the α-amino group is essentially fully protonated (has gained H+) and exists in the –NH3+ state at pH 7.0.
Therefore, the backbone portion of a free amino acid at pH 7 is best represented as:
+NH3-CHR-COO–
When an amino acid is linked up as part of a peptide chain, the situation is different. The α-amino groups and α-carboxylate groups combine to form amide or peptide bonds. When combined as an amide, the α-amino groups and α-carboxylate groups are not free to protonate or deprotonate, so in the peptide bonded state, the amino acid backbone is uncharged:
x-NH-CHR-CO-NH-CHR-CO-NH-CHR-CO-x
Meaning of pKa
The value of pKa tells you where in the pH scale a functional group undergoes protonation or deprotonation, e.g. for glutamate:
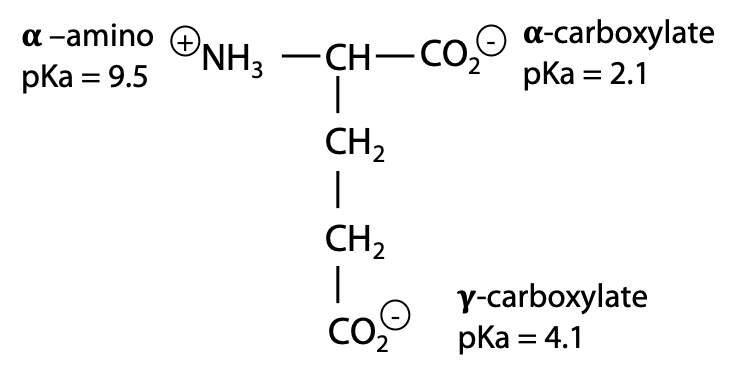
Each group has its own pKa value. The exact value of a group’s pKa depends on its chemical context. The presence of the positive NH3+ near the α-carboxylic acid position favours deprotonation to the negative carboxylate, hence this group is more acidic (lower pKa) than the γ-carboxylate.
When glutamate is part of a peptide chain, the α-amino group is part of a neutral amide bond. Therefore, in the absence of a nearby positive charge, the glutamate side chain carboxylate has pKa = 5.
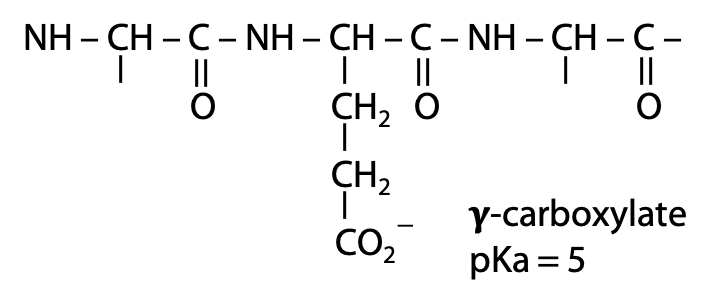
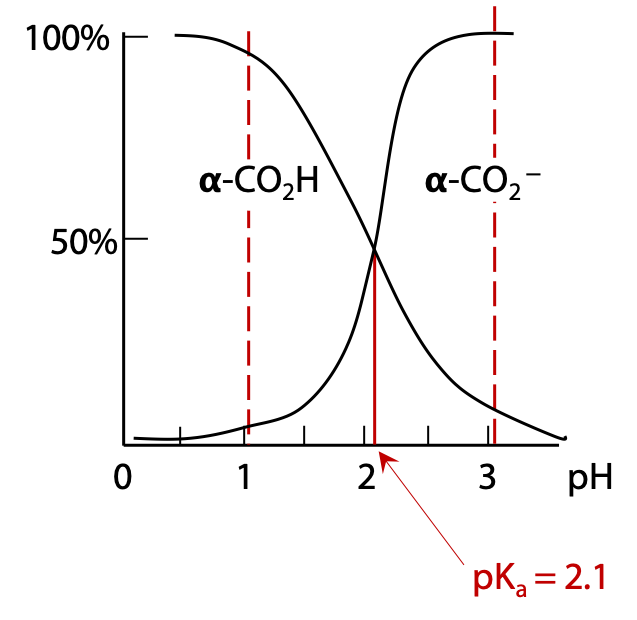
If we start with a sample of free glutamic acid initially at very low pH, all functional groups will be protonated. As pH is increased, each functional group will start to lose H+ when the pH approaches the pKa of that group. For example, deprotonation of the α-carboxylic acid group occurs around pH 2.1. When pH = 2.1, the α-carboxylic acid will be exactly 50% deprotonated (Figure 2.8).
Above pH 3.1, the α-carboxylate group is essentially fully deprotonated.
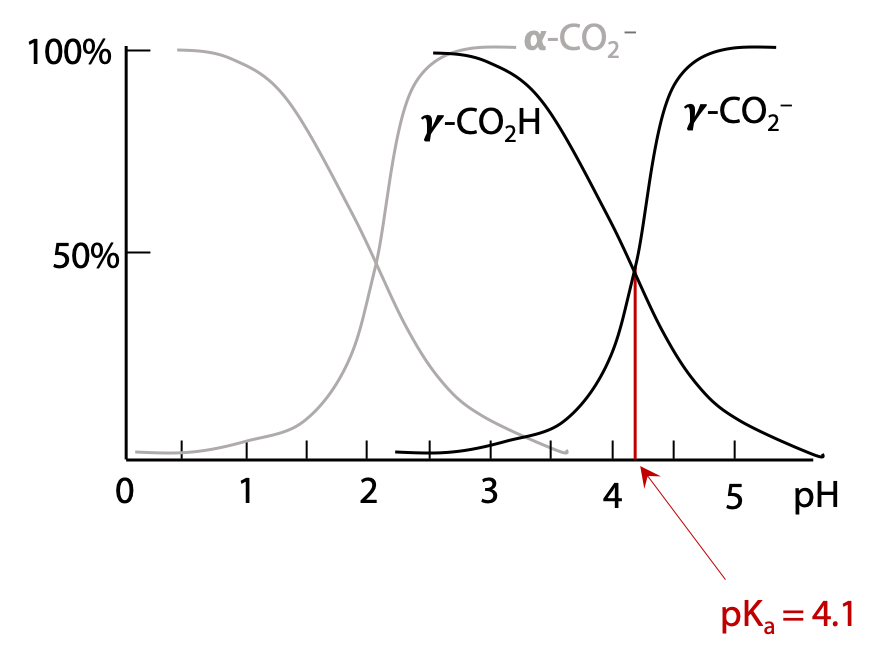
If the pH continues to increase, as it approaches the pKa of the γ-carboxylic acid group, this will deprotonate in turn to yield the γ-carboxylate (Figure 2.9).
Ultimately, if pH is increased much above pH 9, the α-amino group will start to be deprotonated (Figure 2.10).
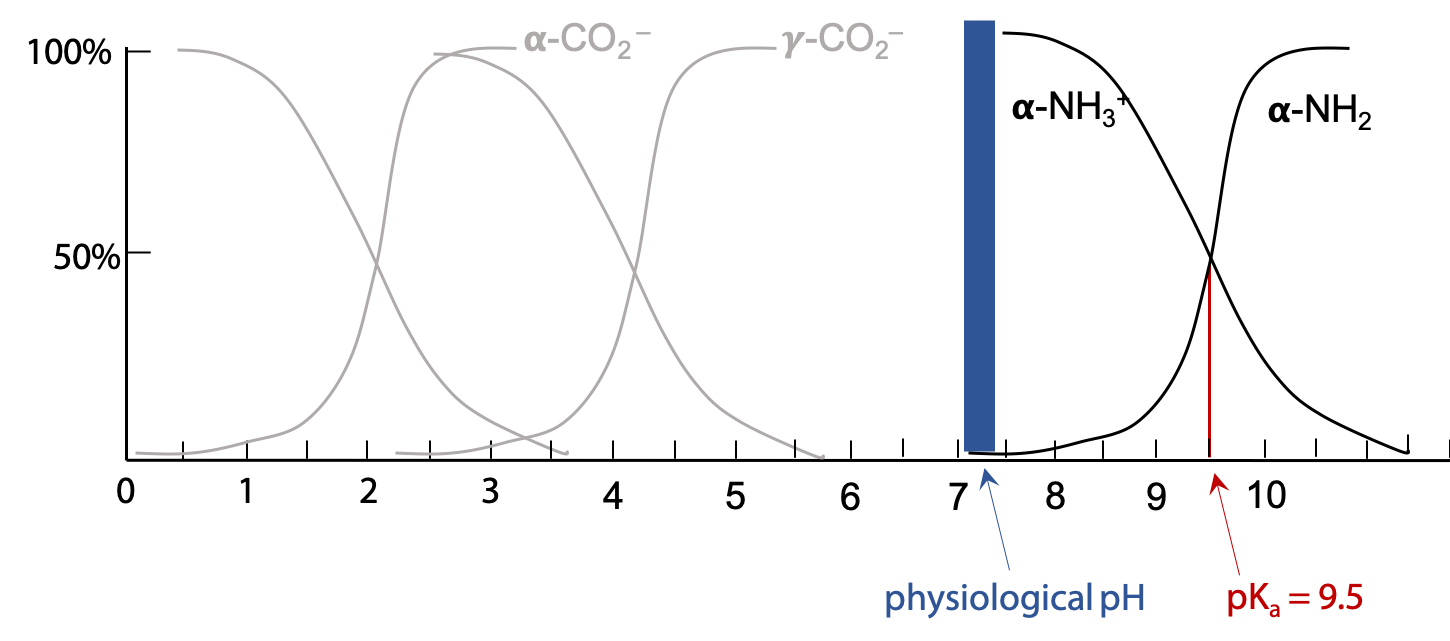
Note that the transition of a given group from fully protonated to fully deprotonated occurs over a narrow range of pH, essentially over a range of 1 pH unit on either side of pKa. This gives us a simple set of rules for determining the ionic state of the functional groups in a molecule at any given pH:
If pH is one unit or more above its pKa, a group may be considered fully deprotonated,
e.g. carboxylate groups with pKa = 2.4 exist as -COO– at pH 7.
If pH is equal to pKa, the group is exactly 50% protonated and 50% deprotonated.
If pH is one unit or more below its pKa, a group may be considered fully protonated,
e.g. amino groups with pKa = 9.6 exist as +NH3– at pH 7.
Hence, it is easy to determine by inspection that glutamate exists with its two carboxylate groups deprotonated and its amino group protonated at physiological pH.
There are seven amino acids with side chains that undergo deprotonation
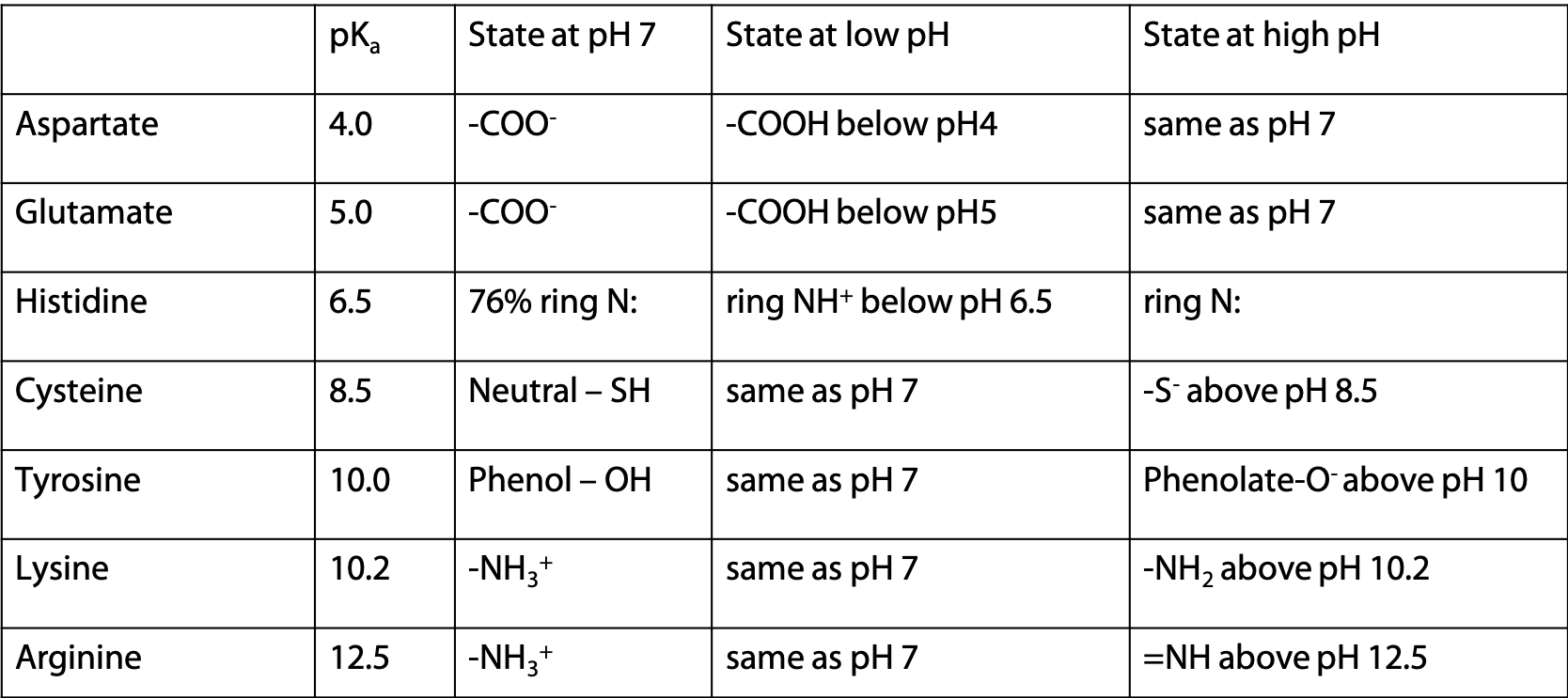
pKa values given are for the side chain of the amino acid in a polypeptide. Values are slightly different for the free amino acid (see Stryer Table 3.4).
Cysteine and Tyrosine were not included as negative in the pyramid table of amino acids, because they are neutral at pH 7.
Important note: These rules can tell you whether a group is protonated or deprotonated, but not immediately tell you whether the group is positively or negatively charged. To determine this, it is necessary to apply a little knowledge of the chemistry of the group:
Groups that ionize on O or S are neutral when protonated and negative when deprotonated.
Groups that ionize on N are positive when protonated and neutral when deprotonated.
No group can go from positive to negative in a single deprotonation step.
Many functional groups contain O, N or S, but do NOT undergo ionization, e.g. alcohols or amides. Simple alcohols (R-OH) such as the side chains of serine or threonine and the amides (R-CONH2) such as the side chains of asparagine or glutamine, do not deprotonate or protonate appreciably in aqueous solution. Their side chains are neither weak acids nor weak bases.
Partial ionization
If pH is less than one unit greater or less than the pKa, we can’t say that a given group is fully protonated or deprotonated. It is still possible to say that the major form is deprotonated if pH is above the pKa and protonated if pH is below the pKa, but the minor form is still present in significant amounts. If we want to be more exact, we can use the Henderson-Hasselbalch equation to determine the exact state of deprotonation.
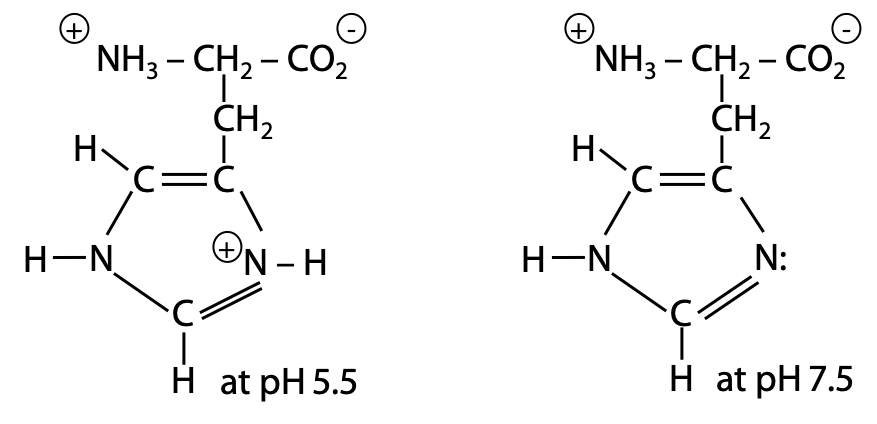
The histidine side chain has pKa = 6.5 (Figure 2.11). At pH 7, the major form is deprotonated, but the pH is not a full unit higher, so it is nowhere near being “fully deprotonated”, and the positively charged form is still present in significant amounts.
What is the degree of deprotonation of the side chain of His (pKa = 6.5) at pH 7.0?
Degree of deprotonation is the fraction of the total that is in the deprotonated state.
(3) ![]()
The Henderson-Hasselbalch equation provides the ratio of deprotonated to protonated His :
(4) ![]()
![]()
![]()
Divide top and bottom by [HisH+]
![Rendered by QuickLaTeX.com \(alpha =\frac{\frac{[His]}{[hisH^+]}}{1+\frac{[His]}{[HisH^+]}}}\(=\frac{3.2}{1+3.2}\(=0.76}](https://ecampusontario.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-32a1da05a42b4776c5bbf3f87e86ee8f_l3.png)
The histidine side chain is 76% deprotonated at pH = 7.0
Relating charge to degree of deprotonation
The degree of deprotonation α tells you what fraction is deprotonated. You need to know something about the chemistry of the functional group to deduce the charge. Since histidine has a N-base in its side chain, the protonated form is positive and the deprotonated form is neutral.
If 76% is deprotonated (charge 0), then 24% must be protonated (charge +1).
Now sum the contributions to average charge:
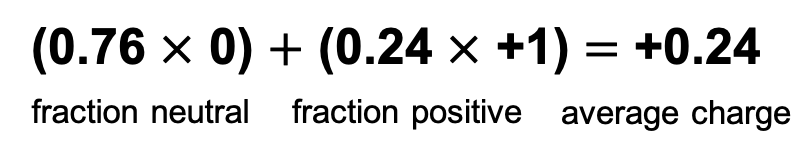
At pH 7.0, Histidine behaves as if it has a side chain charge of +0.24.
Although no one molecule can carry a fractional charge, the +ve HisH+ and neutral His molecules exchange H+ very rapidly (millions of times per second). Averaged over a period of time, each molecule behaves as if it has +0.24 charge.
A calculation similar to the above gives degree of deprotonation = 0.074 for cysteine (pKa = 8.5) at pH 7.4. For cysteine, the side chain states are:
-SH←→ -S– + H+
If 7.4 % is deprotonated single negative -S–, then 92.6% is protonated neutral –SH

Effective charge of cysteine side chain at pH 7.4 = – 0.074. This is low enough that it is usually ignored.
NOTE: The following section will not be covered in lecture, but is provided to refresh your memory of buffers from first year Chemistry.
Buffers
A buffer is a substance present in solution in sufficiently high concentration to control the pH of its environment. This occurs by creating a mixture of the protonated (or weak acid) and deprotonated (or weak base) forms of the buffer such as the mixture is at equilibrium with the desired concentration [H+]. The resulting pH will be close to the pKa of the buffer. Buffering occurs in living cells due to the presence of bicarbonate ions, phosphate ion and phosphate esters, all of which have pKa values close to 7.
In biochemistry labs, two buffers are widely used, chosen because their pKa is close to physiological pH 7-7.4:
Dihydrogen phosphate H2PO4– in equilibrium with HPO42– (pKa = 6.8).
Trishydroxymethylaminomethane or Tris for short, in equilibrium with its protonated form TrisH+ (pKa = 8.08).
