16 ATP as Cellular Energy Currency
The cell needs to make, store and use energy, just as an individual needs to make, store and use money. Without money, a person cannot acquire goods and services. Without energy, a cell cannot synthesize metabolites or carry out physiological functions. What molecule should we use as our “energy currency”? All cells rely primarily on the formation and hydrolysis of phosphorylated chemicals, especially ATP.

The nucleotide triphosphate ATP is the cell’s most important reservoir of free energy available for immediate use. Fritz Lipmann (Figure 16.1) first recognized the significance of ATP and other phosphorylated compounds in bioenergetics. (He also discovered the cofactor Coenzyme A).

ATP is a nucleotide, comprising a base (adenine), a sugar (ribose), and three phosphate groups, which we label alpha, beta, and gamma (Figure 16.2). A 70 kg person uses about 40 kg of ATP during a restful day!! (assuming ~50% efficiency of converting food energy (8400 kJ/2000 kcal/per day) into ATP)
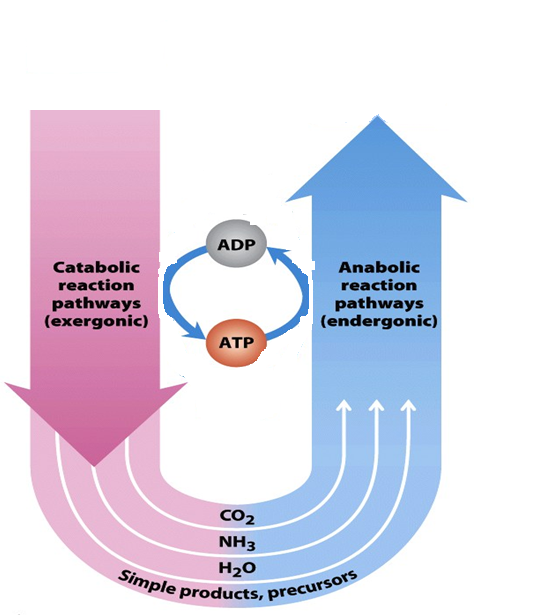
ATP is not a store of chemical energy; rather it’s a link between catabolism and anabolism (Figure 16.3)
ATP’s role in the cell is analogous to that of money in the economy: it is “earned/produced” in exergonic reactions and “spent/consumed” in endergonic ones. Cells breakdown nutrient molecules (catabolism) and use the available free energy to synthesize ATP from ADP. ATP then donates its energy to endergonic processes that require energy as well as synthesis of metabolic intermediates and macromolecules (anabolism), active transport across membranes, mechanical motion etc. ATP turns over (is broken down and synthesized) very rapidly in cells; the typical lifetime of an ATP molecule is measured in seconds to minutes.
The free energy change for hydrolysis of ATP is large and negative
Why did we choose ATP as our energy currency? Most hydrolysis reactions are energetically favourable, but the ΔG value (free energy released by hydrolysis) depends on the nature of the bond being hydrolyzed. Typical values for amides, esters and phosphoesters are about 15-20 kJ per mol. We want to use the hydrolysis of our currency to drive common reactions forward – i.e., Synthesis of proteins, starches and other molecules. This means that we need a more “valuable” currency, i.e., a chemical for which ΔGhydrolysis is considerably higher than 20 kJ per mol. The ΔGhydrolysis for ATP is about 50 kJ/mol. The energy-requiring/ releasing processes take place at the phosphates.
Being energy rich; however, is not the only reason for choosing ATP as the energy currency. The fact that ATP is metabolically available (it’s a RNA building block), kinetically stable (does not undergo hydrolysis unless catalyzed by an enzyme) and chemically versatile (can take part in reactions through many different mechanisms such as group transfer reactions) are important reasons for ATP being chosen as the universal energy currency.
Chemical basis for the large free-energy change associated with ATP hydrolysis

Why does hydrolysis of a phosphoanhydride have such a high ΔG value?
The components of the phosphoanhydride are very negatively charged. Therefore, when hydrolysis releases this electrostatic repulsion among these charges, the negatively charged products fly apart.
The inorganic phosphate product has greater resonance stabilization than ATP does.
The ADP product of hydrolysis rapidly loses another proton (ionizes), which by Le Chatelier’s principle, drives the hydrolysis towards completion (Figure 16.4).
Hydrolysis of ATP
High-energy bonds: People often refer to the beta and gamma phosphoanhydride linkages of ATP as “high-energy bonds”, and even to draw them as “squiggles”: A—P~P~P. This shorthand notation is useful, because it reminds us that hydrolysis of the beta and gamma phosphoanhydride bonds releases a lot more energy than hydrolysis of the alpha phosphate, which is just a phosphate ester. But do not be misled into thinking that the phosphoanhydride bonds themselves are unusual (short/long/ squiggly). The energy is not associated with the bond, instead, with the overall hydrolysis reaction; it is a property of the ATP molecule, not a property of the bond itself.
There are two “high-energy bonds” (phosphoanhydride linkages) in ATP, the beta and gamma phosphoanhydride linkages. Either could be hydrolyzed. Indeed, some enzymes catalyze one mode of hydrolysis and some choose the other.
-
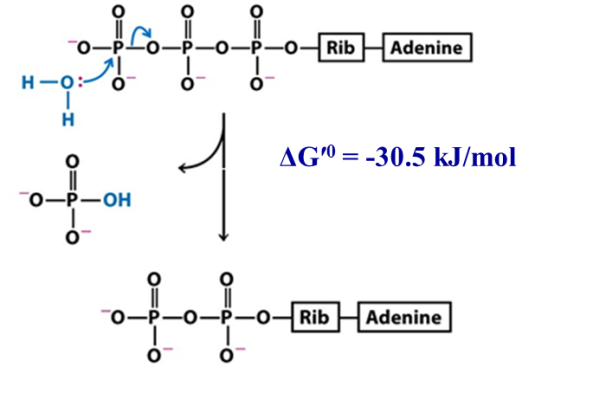
Figure 16.5 The hydrolysis of ATP yields ADP and Pi Hydrolysis of the link between gamma and beta phosphate (nucleophilic attack of the gamma phosphate) yields ADP and Pi, which we already discussed (Figure 16.5):
ATP → ADP + Pi
Figure 16.6 Pyrophosphate 2. Hydrolysis of the alpha-beta linkage (nucleophilic attack of the alpha phosphate) yields AMP and pyrophosphate (PPi) (Figure 16.6). This compound is a dimer of phosphoric acid moieties linked by a phosphoanhydride bond, and it is still a “high-energy” compound.
ATP → AMP + PPi
In the cell, pyrophosphate usually undergoes further hydrolysis to give two moles of Pi per mole of pyrophosphate, in a reaction catalyzed by the enzyme pyrophosphatase.
PPi →2Pi
pyrophosphatase
Net: ATP → AMP + 2Pi
Overall, the hydrolysis of ATP to AMP and PPi is tantamount to the complete hydrolysis of ATP to AMP + 2 Pi, which is, in other words, the hydrolysis of both of the high-energy bonds in ATP. In terms of effect, the free energy of hydrolysis of ATP to AMP + PPi is twice as large as the free energy of hydrolysis of ATP to ADP + Pi.
How does hydrolysis of ATP drive energetically-unfavourable reactions?
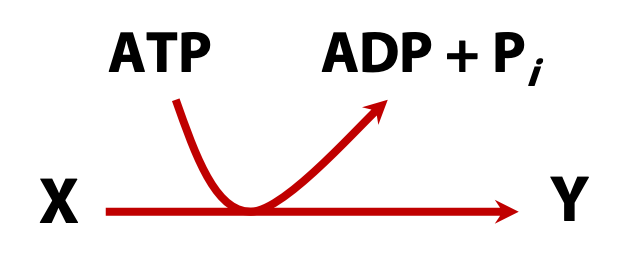
This is a very important concept. You are going to see, in many times, the notation shown in Figure 16.7 indicates that reaction (such as a biosynthesis, a transport process or some other energy-requiring step) is “driven by the hydrolysis of ATP”. In a thermodynamic sense, this means that the free energy needed to drive the reaction forward (overcoming its positive free energy change) is supplied by ATP hydrolysis.
This is true; it is equivalent to say that the electrical energy supplied through the mains provides the energy needed to turn the blades of a food processor. Another example is that the chemical energy in gasoline provides the energy needed to drive a car forward (Figure 16.8). It is a valid thermodynamic statement, but it tells us nothing about mechanism. So, whenever you see that “driven by the hydrolysis of ATP” arrow notation, you must ask yourself, “What is the chemical mechanism that allows ATP hydrolysis to drive this reaction forward?”
How ATP drives an energy-requiring reaction: an example

Glutamine synthetase provides a nice example of the usage of ATP energy in biochemistry. Glutamine is one of the twenty amino acid building blocks of protein. It is chemically similar to glutamate, another of the building blocks. In fact, glutamine is synthesized from glutamate by the enzyme-catalyzed condensation of ammonia with the gamma-carboxylic acid moiety of glutamate. This condensation gives the corresponding amide: glutamine (top reaction). This reaction is energetically “uphill”, while the reverse reaction, hydrolysis of glutamine to glutamate and ammonia, is spontaneous. The cell drives the synthesis of glutamine forward by coupling the reaction to ATP hydrolysis, as illustrated by the ATP ADP + Pi notation (Figure 16.9).
The mechanism of the enzyme-catalyzed synthetic reaction, which is coupled to ATP hydrolysis, is known in considerable detail. The reaction occurs in two steps. ATP is a reactant, even though it does not appear in the stoichiometry “glutamate + ammonia glutamine”. In the first step, ATP reacts with glutamate to produce a covalent intermediate, a mixed anhydride of phosphate and glutamate. In the second step, ammonia, as a nucleophile, reacts with the electrophilic carbonyl carbon atom. Pi, as the leaving group, is displaced.
We have already discussed some of the reasons why ATP is a suitable energy currency for the cell. Now we will see another important aspect of ATP biochemistry. Phosphorylated compounds such as ATP are chemically versatile: the phosphate group can participate in a variety of chemical reactions with common organic functional groups. Our currency is accepted wherever we wish to spend it.
In addition to the phosphoryl group, ATP can also transfer a pyrophosphoryl (PPi) or an adenylate (AMP) moiety to a substrate or to an amino acid residue of an enzyme to help to drive reactions forward. Therefore, it is obvious that ATP provides energy not by simple hydrolysis but through group transfer.

We have said that hydrolysis of phosphoanhydrides has high negative ΔG values. Figure 16.10 shows the relative ΔG values for hydrolysis of some biochemically-important phosphorylated molecules and for acetyl CoA. (The ΔG scale is flipped, with negative values at the top, so that the “highest-energy” compounds are at the top of the hill.) At the bottom – the zero value – is inorganic phosphate, which can’t be hydrolyzed at all, because it’s the product of hydrolysis. Simple phosphate esters of alcohols, like sugar phosphates are “low-energy” phosphate bonds. The hydrolysis of these compounds is energetically comparable to the hydrolysis of simple esters or amides. ATP, as we have said, is a “high-energy” phosphate compound. Above ATP, there are some “super-high-energy” phosphate compounds, like phospho-enol-pyruvate and creatine phosphate. Creatine phosphate stores energy in muscles. “Super-high-energy” phosphate compounds can generate ATP directly, by the energetically favourable transfer of the phosphate group to ADP.
Self-assessment Questions
1.
2.
3.
