19 Adenosine-Containing Cofactors
There are some enzymes require the help of additional chemical compounds (cofactors) to carry out their functions. These could be:
Inorganic ions: Fe2+, Mg2+, Mn2+, Zn2+, Cu2+
Cofactors: complex organic or metalloorganic compounds that act as transient carriers of specific functional groups.
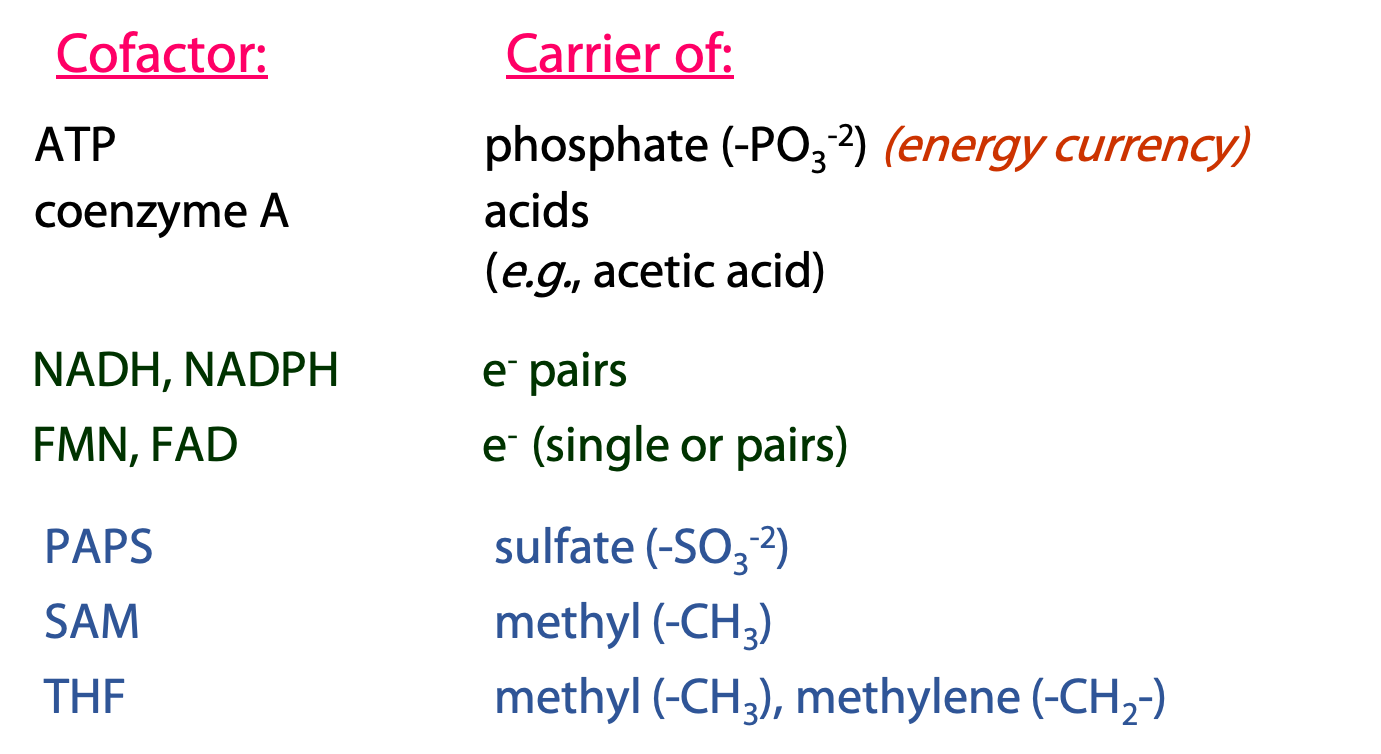
Over and over again in metabolism, we see the same types of reactions, with the same coenzymes participating. Many of these cofactors are derivatives of adenosine (Figure 19.1) – another indication of the primary role of RNA nucleotides in the evolution of the first cells. We are going to discuss the cofactors shown on the top of this list. The ones on the bottom (PAPS, SAM, THF) will be left for later courses.
ATP: carrier/donor of phosphate groups
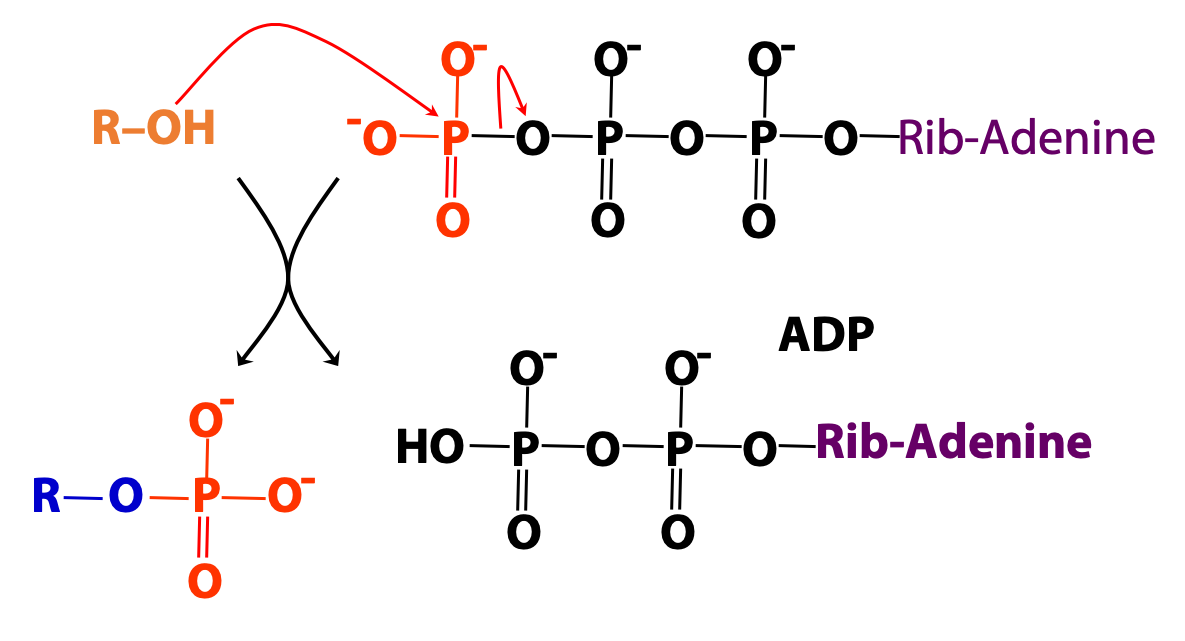
We introduced ATP as the cellular energy currency. ATP can also be regarded as the metabolic carrier of phosphate groups, which can be donated to (i.e., used to phosphorylate) many types of molecules – sugars, lipids, proteins, etc (Figures 18.2 and 18.3).

Coenzyme A (CoA/CoASH): carrier of acyl (acid) groups

Coenzyme A was discovered by Fritz Lipmann (see Figure 16.1) , who shared the 1953 Nobel Prize in Physiology or Medicine with Hans Krebs. We often abbreviate coenzyme A as “CoASH”, to emphasize the presence of the thiol (-SH) group.

Coenzyme A is derived from the vitamin pantothenic acid (Vit B5, Figure 18.5)
CoASH forms thioester derivatives with organic acids (R-COOH).

A thioester is simply the sulfur analogue of an ester (oxy-ester), which is formed by the condensation of an acid and an alcohol (Figure 18.6):
Alcohol + Acid Ester
Thiol + Acid Thioester
*An acid functional group within a molecule is referred to an “acyl” group. Therefore, any coenzyme-A thioester derivatives are called “acyl CoA”. In the specific case of acetic acid (H3C-COOH), the corresponding coenzyme A derivative is called “acetyl CoA”, which is often written as “CoASAc” (“Ac” standing for “acetyl” rather than the generic “acyl”). Later, we will look at other acyl CoA molecules, such as palmitoyl CoA, which is the CoA thioester of palmitic acid.
Acetyl coenzyme A is probably the most central and important molecule in metabolism. It is formed by the metabolism of sugars and lipids, and is the source of carbon atoms entering the citric acid cycle. CoASH is the biological carrier of acetate, which is nature’s favourite building block for the assembly of more complex molecules. Fatty acids and steroids, for example, are synthesized from acetyl CoA.
Nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide phosphate (NADP+), Flavin adenine dinucleotide (FAD) and Flavin mono nucleotide (FMN): The universal electron carriers
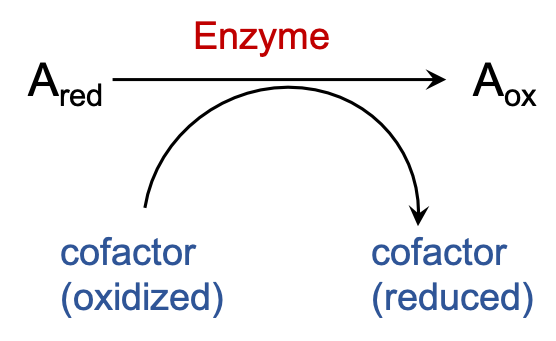 There are many enzymes that carry out oxidation of different substrates rely on a few cofactors to act as their electron acceptor. The electrons that are removed from the substrates are transferred onto these cofactors to reduce them and consere the energy of oxidation.
There are many enzymes that carry out oxidation of different substrates rely on a few cofactors to act as their electron acceptor. The electrons that are removed from the substrates are transferred onto these cofactors to reduce them and consere the energy of oxidation.
NAD(P)+ and NAD(P)H: The Pyridine Nucleotides

The pyridine nucleotides are NAD+ and NADP+ (in their oxidized forms), NADH and NADPH (in their reduced forms). These molecules are the most important biological reducing and oxidizing agents. They only differ in the presence (NADP+/NADPH) or absence (NAD+/NADH) of the phosphate at the 2 position of the adenosine moiety (Figure 18.7) . Note the unusual (compared to nucleic acids) pyrophosphate linkage between the 5 –OH groups of the two nucleotide moieties.
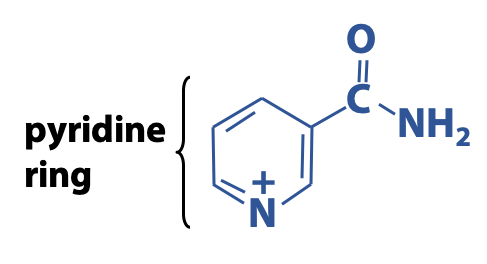
The nicotinamide moiety of NAD+ and NADP+ is derived from the vitamin niacin (B3) (Figure 18.8).
Redox chemistry of the pyridine nucleotides

The redox properties of NAD+ and NADP+ are so similar that we can discuss them together – that’s why it is written as “NAD(P)+”. Why do we write “NAD+” rather than simply “NAD”? The “+” sign represents the positive charge on the N atom of the oxidized nicotinamide ring. (The overall electrical charge on the molecule is negative, because of the phosphates, but our focus here is on the redox chemistry of the pyridine ring.)
Redox reactions occur at the nicotinamide ring (Figure 18.9). The oxidized nicotinamide ring undergoes a 2e– + H+ reduction (or addition of H–, or “hydride ion”). Therefore, the product is NAD(P)H, with a neutral reduced nicotinamide ring – i.e., bearing no charge on the N atom. Hence, we can write:
NAD+ + 2e– + H+ → NADH
NADP+ + 2e– + H+ → NADPH
Both the electrical charges and the H atoms are balanced. Equivalently (as shown in the above figure), we can write:
NAD+ + 2e– + 2H+ → NADH + H+
This represents a transfer of two neutral H atoms, which would occur in the oxidation of an alcohol to a ketone as a typical NAD+– mediated reaction. This explains why we often see the expression “NADH + H+” appearing in biochemical equations. The point is always to keep the charges and the H atoms balanced.
The single phosphate group that distinguishes NAD+/ NADH from NADP+/ NADPH makes a big difference: most enzymes can easily distinguish between the two forms of pyridine nucleotides, and they play very different roles in the cell. As a general rule (there are a few exceptions), NAD+ is used as the oxidizing agent in catabolic processes like fatty acid oxidation, and the resulting NADH is reoxidized via the electron transport chain to generate energy. In contrast, NADPH is used as the reducing agent in biosynthesis (e.g. Fatty acid synthesis, steroid synthesis etc.)
FAD/FADH2 and FMN/FMNH2: Flavin Nucleotides
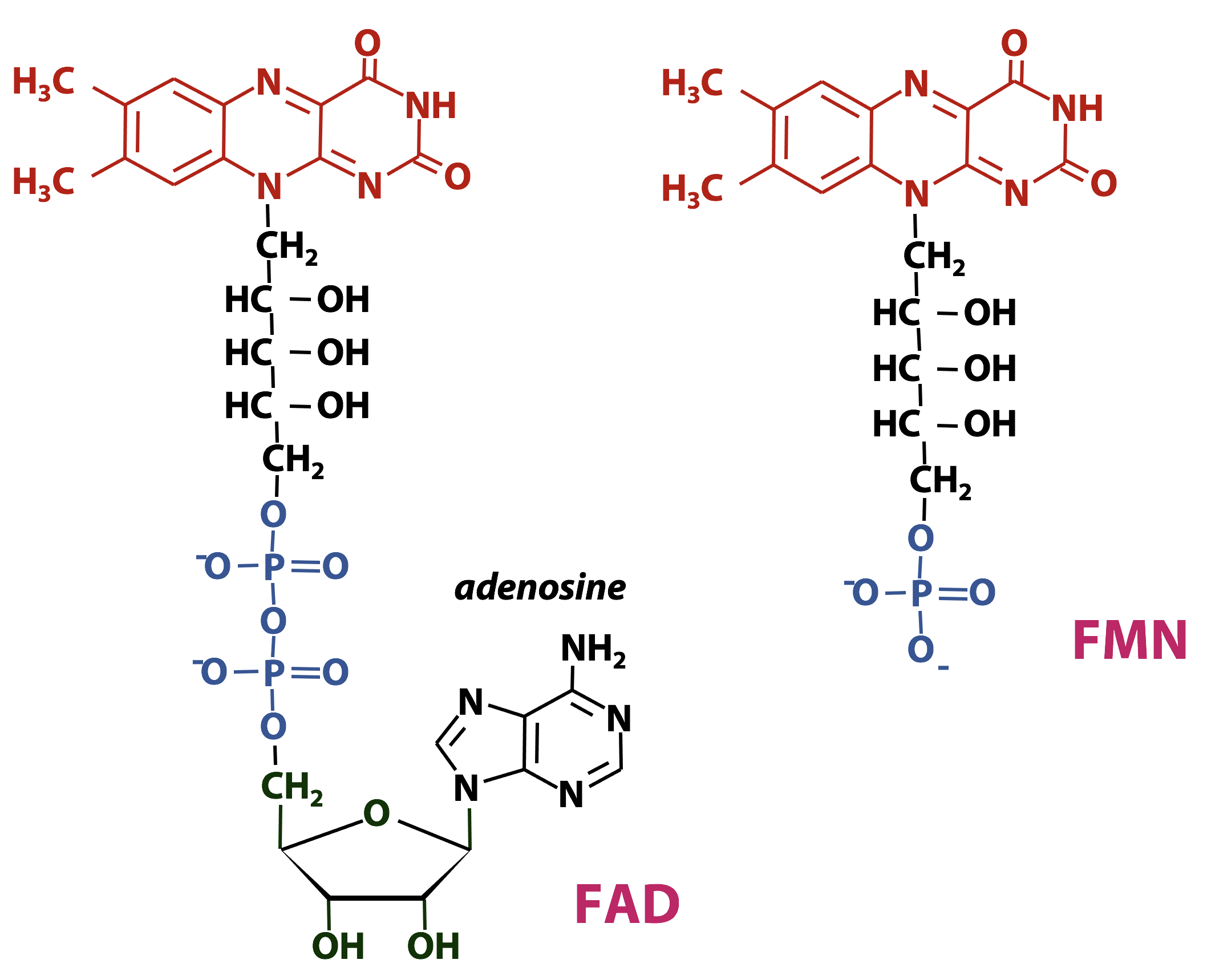
Flavin nucleotides are important redox cofactors in biochemistry. The name flavin was coined from the Latin word flavus, which means “yellow”. The flavin ring system is an extended conjugated aromatic system (Figure 18.10), so flavins absorb visible light strongly – flavins and flavoproteins are bright yellow- or orange-coloured. The chemical name for the flavin ring is “dimethyl-isoalloxazine”.
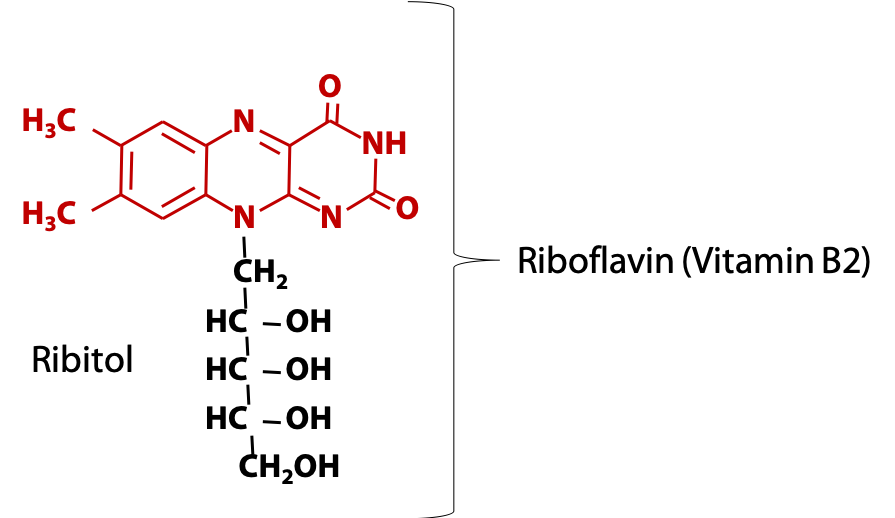
Animals obtain the flavin ring in the form of vitamin B2, riboflavin (Figure 18.11), which consists of the flavin ring plus the sugar-alcohol ribitol.
Flavin nucleotides usually act as prosthetic groups: they are found tightly bound to the enzyme.
Redox chemistry of the flavin nucleotides
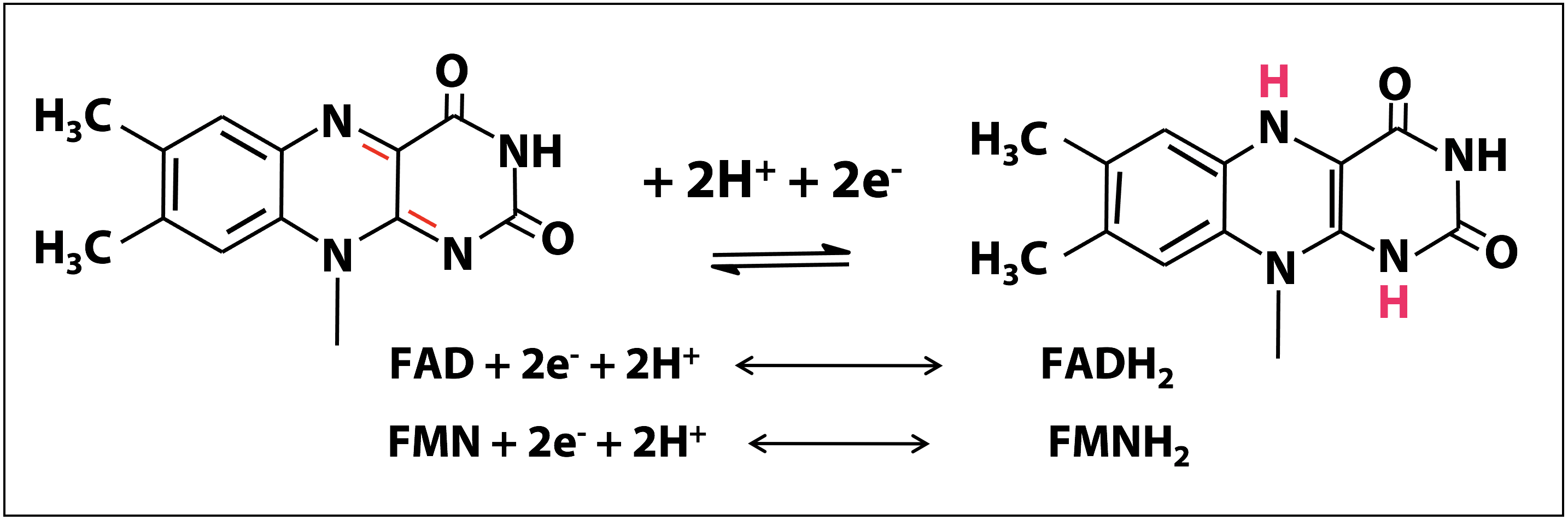
The redox chemistry of FAD and FMN takes place on the flavin ring. Flavin nucleotides can accept either one or two electrons in the form of one or two hydrogen atoms (each atom, an electron plus a proton) from substrates undergoing oxidation. In Figure 18.12, the two electron redox reactions are shown. The fully reduced forms of the nucleotides are FADH2 and FMNH2 and when only one electron is accepted, they form the stable semiquinone radical forms of FADH. and FMNH.. For these cofactors, neither the oxidized nor the reduced form of the ring is charged. So we can just write:
FAD + 2e– + 2H+ ↔ FADH2
Enzymes that catalyze oxidation-reduction reactions with the help of either FMN or FAD are known as flavoprotiens. Due to the ability of FMN and FAD to participate in either one or two electron transfers, flavoproteins are involved in a greater diversity of reactions than the NAD(P)-linked dehydrogenases.
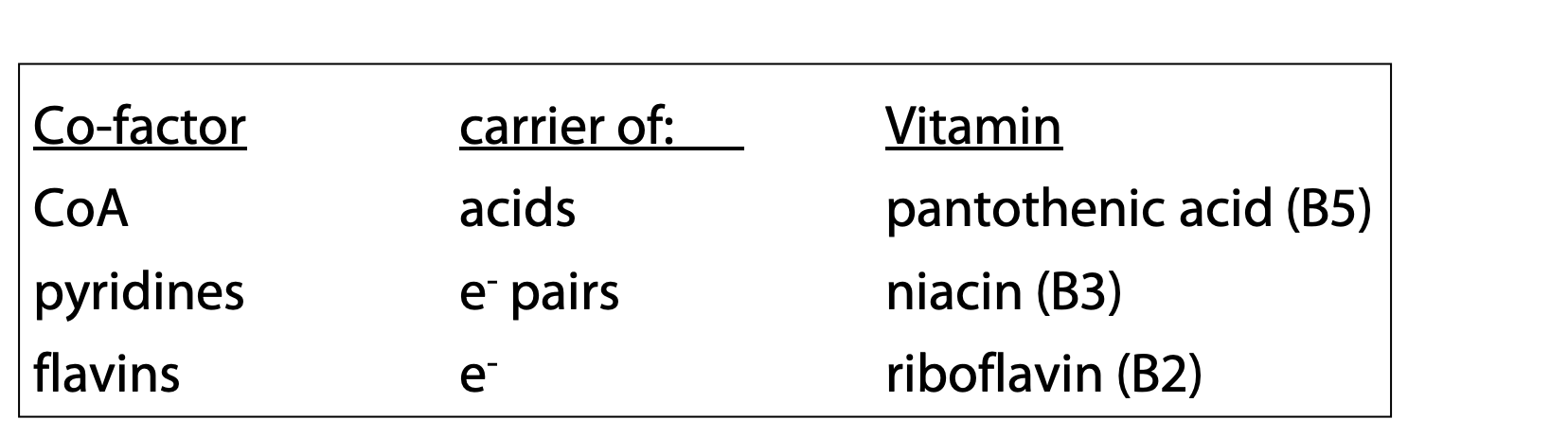
B-Vitamin cofactors: Summary