30 7.4 – Effects of Fecal Microbiota Transplant in Crohn’s Disease
This study aimed to assess the efficacy of multiple fresh FMT in the treatment of patients with Crohn’s disease that were also co-morbid for an inflammatory mass within the digestive system. The primary outcome was to assess clinical response via the Harvey Bradshaw Index (HBI), and secondary outcome measures looked at improvement in size of inflammatory masses via magnetic resonance imaging (MRI), and adverse effects.
Important definitions:
clinical response: > 50% reduction from baseline symptoms
clinical remission: HBI score < 3, which is classified as normal.
sustained remission: no relapse of symptoms at any point after initial treatment, and subsequently no FMT treatment required
Primary Outcome Results:
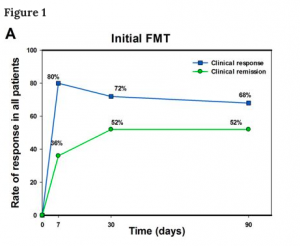
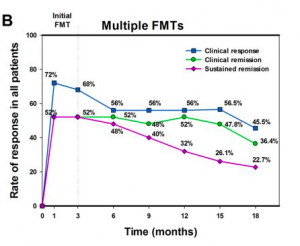
Figure 1a. showcases the results of the initial FMT treatment on a short term follow up, where 20/25 patients achieved clinical response, with 9 achieving clinical remission 7 days after the treatment. 90 days after assessment, 17/25 patients maintained clinical response and 13/25 patients had achieved clinical remission. Figure 1b., follows up with patients over a longer time, across multiple FMT treatments up to 18 months after therapy; 3 patients dropped out at a point between 12 months and 18 months). It is seen that while the rates of clinical response, remission and sustained remission all see a decrease as the assessment points go on, 10/22 patients remain qualified for clinical response, 8/22 achieve clinical remission, and 5/22 patients maintain a sustained remission. These results suggest that FMT treatment may be a potential therapy short term therapy for Crohn’s disease that merits further research, especially with controlled clinical trials.
Secondary Outcome Results:
Inflammatory mass imaging:
21 of 25 patients underwent the imaging evaluation of inflammatory mass at baseline and 3 months after FMT. 2/21 patients achieved healing, 15/21 patients achieved improvement, 2/21 patients showed no improvement and 2/21 patient had worsened conditions.
Safety and adverse events:
The authors reported that there were no severe adverse events associated with FMT during the initial 3 month assessment and the follow up. Most common adverse effects reported were fever, self-limiting diarrhea. An uncommon adverse event was transient anal pain after FMT that self-resolved.
Shh-It’s Complicated:
There were a few aspects of this study that can call into question its accuracy. Primarily, it is an open label pilot study, however, there are a few protocols that took place over the course of the experiment that could lead to inconsistencies such as the author’s choosing not to homogenize fecal samples from healthy donors and instead matching donors to patients, and patients being allowed a longer time to undergo repeated FMTs due to inconvenience or satisfactory samples. While it is important to keep patient considerations in mind, it is also imperative in science to follow a stringent procedure so as not to introduce additional modes of error in experiments. It is very important to follow a road of diligence and scientific soundness.
