9.4 Future Directions
Limitations
Several major questions still remain about the cancer initiating cells, specifically with regards to the niche environment which they arise in. Initiation could be a downstream effect of neural crest signalling pathways, stress pathways, irradiation or oxidative damage. Furthermore, researchers are still unsure what causes cancer initiation in cell A as opposed to cell B, and how the crestin gene initiates the cancer development process in the first place. Answering these questions can help derive the targeting mechanisms for cancer initiation.

Future Directions
From an experimental point of view, as mentioned before in section 2 other model organism such as transgenic mouse models are considered the gold standard for cancer research. Mice have been shown to be useful in defining mutations that drive cancer initiation and progression (Zhang et al.). Mouse models helps overcome the limitation present in cell lines which include the inability to examine physiological interactions among tumor cells and their environment in vivo (Zhang et al.). Lastly, the tumors generated are genetically and morphologically much more similar to humans. Therefore it is essential to prove the results found in the zebrafish models in mouse models.
From a diagnostic point of view, novel therapeutic techniques could use the turning on of these neural crest genes to detect malignant moles. Surgery can then be performed to excise the mole and prevent it from metastasizing. Furthermore, developing a therapeutic cream which can turn off genes that prevent the neural crest from being re-expressed in the first place could also be a potential treatment option.
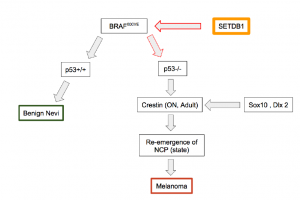
In previous studies it has been shown that upstream transcription regulators of BRAF600VE plays a significant role in accelerating zebrafish melanoma (Ceol et al.). Moreover, SETDB1 has shown to regulate histone methylation, gene silencing, and transcriptional repression . Overexpression of SETDB1 protein is common in human melanomas, making the tumors over aggressive (Ceol et al.). That being said, SETDB1 over-expression could be used as a new biomarker for melanoma. SETDB1’s effects on histone methyltransferase activity could also have important implications in other biologically related subset of cancers. Thus, SETDB1, or proteins that regulate SETDB1 activity, could represent a therapeutic target.