9.3 Zebrafish models of Melanoma
Learning Objectives
What makes Zebrafish a good model for human melanomas?
What is the MiniCoopR system?
What is the role of SOX10?
Why is the Zebrafish a good model for human melanomas?
Zebrafish are an important model organism for studying cancer metastasis in vivo. In addition to being vertebrates, they possess many genes that are similar to those involved in human cancer progression (Van Rooijen et al, 403). An example of this is the BRAFV600E oncogene, which exists in both humans and zebrafish (Kaufman et al, 3). In cases of human melanoma metastasis, mutations of BRAF V600E, which cause it to be overexpressed, have been observed. In vivo studies with zebrafish have lead researchers to discover that in order for this mutation to cause tumor formation, a deficiency in the cell growth suppressor p53 must also be present (Van Rooijen et al, 404). This combination creates what has come to be known as a “cancerized background”, and can be easily replicated within zebrafish (Kaufman et al, 3).
The MiniCoopR system is a common method employed by researchers to create transgenic zebrafish. It involves the creation of a plasmid, using the mifta promoter sequence, which works exclusively in skin cells (Heilmann et al, 4273). EGFP or fluorescent green protein is also added to the construct, to allow for easy visual detection of melanoma cells (Kaufman et al, 3). Finally, the genetic construct is implanted into zebrafish embryos, that have an overexpression of BRAF V600E oncogene and a lack of p53 tumor suppressor. This is illustrated in figure 1 (Biorender).
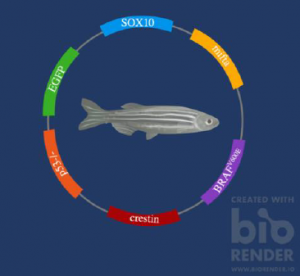
Figure 1. shows the genetic components of transgenic zebrafish that make them good candidates for melanoma modelling. (Made with Biorender).
Several genetic components that are common to both zebrafish and humans have been identified through in vivo and in vitro studies, and have been found to play a central role in melanoma growth. SOX10 and PAX3 are genes that play central roles in tissue development during embryogenesis (Kaufman et al, 4). For example, in a study by Kaufman et al., SOX10 was excised from the zebrafish using CRISPR/Cas-9, which caused a significant decrease in tumor growth (5). When this gene was overexpressed, the opposite was observed: tumor initiation and proliferation were both rapid (Kaufman et al, 4). Additionally, crestin is a gene unique to zebrafish, and has been identified as a crucial component for melanoma onset (Van Rooijen et al, 406). Crestin is expressed naturally in the neural crest progenitor cells of zebrafish for the first 72 hours after fertilization, after which their activity is naturally terminated (Kaufman et al, 3). Kaufman et al. found that melanomas can reinitiate the proliferative activity of neural crest progenitor cells by causing re-expression of crestin (3). A human equivalent of crestin has yet to be identified (Van Rooijen et al, 406).
Results
The experiments conducted by Kaufman et al. further support the information presented above. The researchers first fluorescently labelled crestin with EGFP so that they could visualize its location and expression levels in melanoma (Kaufman et al, 3). Importantly, the expression pattern of EGFP/crestin consistent with that previously identified by in situ hybridization, thus ensuring accuracy of the results. This can be seen in figure 2.
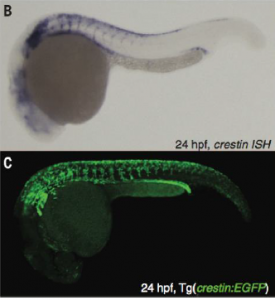
Figure 2 is a comparison between the ISH and fluorescence microscopy images generated by Kaufman et al.
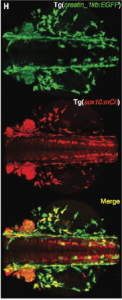
As mentioned above Kaufman et al. identified SOX10 as an important transcription factor that regulates tissue growth as well as tumor progression (4). This was followed by the fluorescent labeling of SOX10 with mCherry to gain insight if it regulates expression of crestin (Kaufman et al, 3). In figure 3 you can see that both crestin and SOX10 act in similar areas, suggesting that they influence each other in some way.
Finally figure 4 shows the expression patterns of zebrafish after SOX10 or PAX3 were knocked out. The researchers did this in order to determine which transcription factor was responsible for the activity of neural crest progenitor cells and thus crestin expression. When SOX10 was repressed, there was a reduction in neural crest progenitor cell activity, which indicated that this was the transcription factor controlling expression levels (Kaufman et al, 4). This was visualized with ATAC and ChIP-sequencing.
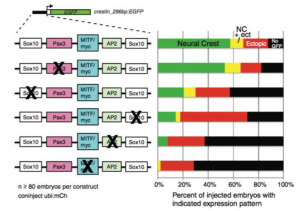
Figure 4 shows the expression patterns in transgenic zebrafish when SOX10 and PAX3 were repressed.
