The Adaptive Immune Response: T lymphocytes and Their Functional Types
Learning Objectives
By the end of this section, you will be able to:
- Explain the advantages of the adaptive immune response over the innate immune response
- List the various characteristics of an antigen
- Describe the types of T cell antigen receptors
- Outline the steps of T cell development
- Describe the major T cell types and their functions
Innate immune responses (and early induced responses) are in many cases ineffective at completely controlling pathogen growth. However, they slow pathogen growth and allow time for the adaptive immune response to strengthen and either control or eliminate the pathogen. The innate immune system also sends signals to the cells of the adaptive immune system, guiding them in how to attack the pathogen. Thus, these are the two important arms of the immune response.
The Benefits of the Adaptive Immune Response
The specificity of the adaptive immune response—its ability to specifically recognize and make a response against a wide variety of pathogens—is its great strength. Antigens, the small chemical groups often associated with pathogens, are recognized by receptors on the surface of B and T lymphocytes. The adaptive immune response to these antigens is so versatile that it can respond to nearly any pathogen. This increase in specificity comes because the adaptive immune response has a unique way to develop as many as 1011, or 100 trillion, different receptors to recognize nearly every conceivable pathogen. How could so many different types of antibodies be encoded? And what about the many specificities of T cells? There is not nearly enough DNA in a cell to have a separate gene for each specificity. The mechanism was finally worked out in the 1970s and 1980s using the new tools of molecular genetics.
Primary Disease and Immunological Memory
The immune system’s first exposure to a pathogen is called a primary adaptive response. Symptoms of a first infection, called primary disease, are always relatively severe because it takes time for an initial adaptive immune response to a pathogen to become effective.
Upon re-exposure to the same pathogen, a secondary adaptive immune response is generated, which is stronger and faster than the primary response. The secondary adaptive response often eliminates a pathogen before it can cause significant tissue damage or any symptoms. Without symptoms, there is no disease, and the individual is not even aware of the infection. This secondary response is the basis of immunological memory, which protects us from getting diseases repeatedly from the same pathogen. By this mechanism, an individual’s exposure to pathogens early in life spares the person from these diseases later in life.
Self Recognition
A third important feature of the adaptive immune response is its ability to distinguish between self-antigens, those that are normally present in the body, and foreign antigens, those that might be on a potential pathogen. As T and B cells mature, there are mechanisms in place that prevent them from recognizing self-antigen, preventing a damaging immune response against the body. These mechanisms are not 100 percent effective, however, and their breakdown leads to autoimmune diseases, which will be discussed later in this chapter.
T Cell-Mediated Immune Responses
The primary cells that control the adaptive immune response are the lymphocytes, the T and B cells. T cells are particularly important, as they not only control a multitude of immune responses directly, but also control B cell immune responses in many cases as well. Thus, many of the decisions about how to attack a pathogen are made at the T cell level, and knowledge of their functional types is crucial to understanding the functioning and regulation of adaptive immune responses as a whole.
T lymphocytes recognize antigens based on a two-chain protein receptor. The most common and important of these are the alpha-beta T cell receptors (Figure 21.15).
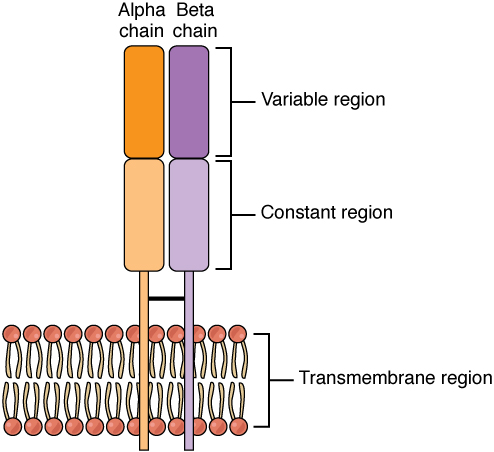 Figure 21.15 Alpha-beta T Cell Receptor Notice the constant and variable regions of each chain, anchored by the transmembrane region.
Figure 21.15 Alpha-beta T Cell Receptor Notice the constant and variable regions of each chain, anchored by the transmembrane region.
There are two chains in the T cell receptor, and each chain consists of two domains. The variable region domain is furthest away from the T cell membrane and is so named because its amino acid sequence varies between receptors. In contrast, the constant region domain has less variation. The differences in the amino acid sequences of the variable domains are the molecular basis of the diversity of antigens the receptor can recognize. Thus, the antigen-binding site of the receptor consists of the terminal ends of both receptor chains, and the amino acid sequences of those two areas combine to determine its antigenic specificity. Each T cell produces only one type of receptor and thus is specific for a single particular antigen.
Antigens
Antigens on pathogens are usually large and complex, and consist of many antigenic determinants. An antigenic determinant (epitope) is one of the small regions within an antigen to which a receptor can bind, and antigenic determinants are limited by the size of the receptor itself. They usually consist of six or fewer amino acid residues in a protein, or one or two sugar moieties in a carbohydrate antigen. Antigenic determinants on a carbohydrate antigen are usually less diverse than on a protein antigen. Carbohydrate antigens are found on bacterial cell walls and on red blood cells (the ABO blood group antigens). Protein antigens are complex because of the variety of three-dimensional shapes that proteins can assume, and are especially important for the immune responses to viruses and worm parasites. It is the interaction of the shape of the antigen and the complementary shape of the amino acids of the antigen-binding site that accounts for the chemical basis of specificity (Figure 21.16).
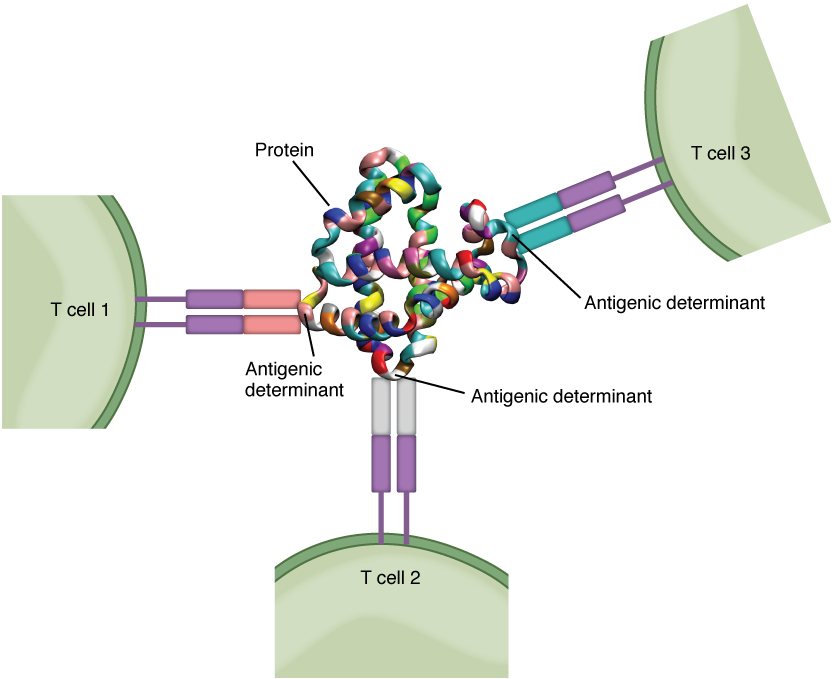 Figure 21.16 Antigenic Determinants A typical protein antigen has multiple antigenic determinants, shown by the ability of T cells with three different specificities to bind to different parts of the same antigen.
Figure 21.16 Antigenic Determinants A typical protein antigen has multiple antigenic determinants, shown by the ability of T cells with three different specificities to bind to different parts of the same antigen.
Antigen Processing and Presentation
Although Figure 21.16 shows T cell receptors interacting with antigenic determinants directly, the mechanism that T cells use to recognize antigens is, in reality, much more complex. T cells do not recognize free-floating or cell-bound antigens as they appear on the surface of the pathogen. They only recognize antigen on the surface of specialized cells called antigen-presenting cells. Antigens are internalized by these cells. Antigen processing is a mechanism that enzymatically cleaves the antigen into smaller pieces. The antigen fragments are then brought to the cell’s surface and associated with a specialized type of antigen-presenting protein known as a major histocompatibility complex (MHC) molecule. The MHC is the cluster of genes that encode these antigen-presenting molecules. The association of the antigen fragments with an MHC molecule on the surface of a cell is known as antigen presentation and results in the recognition of antigen by a T cell. This association of antigen and MHC occurs inside the cell, and it is the complex of the two that is brought to the surface. The peptide-binding cleft is a small indentation at the end of the MHC molecule that is furthest away from the cell membrane; it is here that the processed fragment of antigen sits. MHC molecules are capable of presenting a variety of antigens, depending on the amino acid sequence, in their peptide-binding clefts. It is the combination of the MHC molecule and the fragment of the original peptide or carbohydrate that is actually physically recognized by the T cell receptor (Figure 21.17).
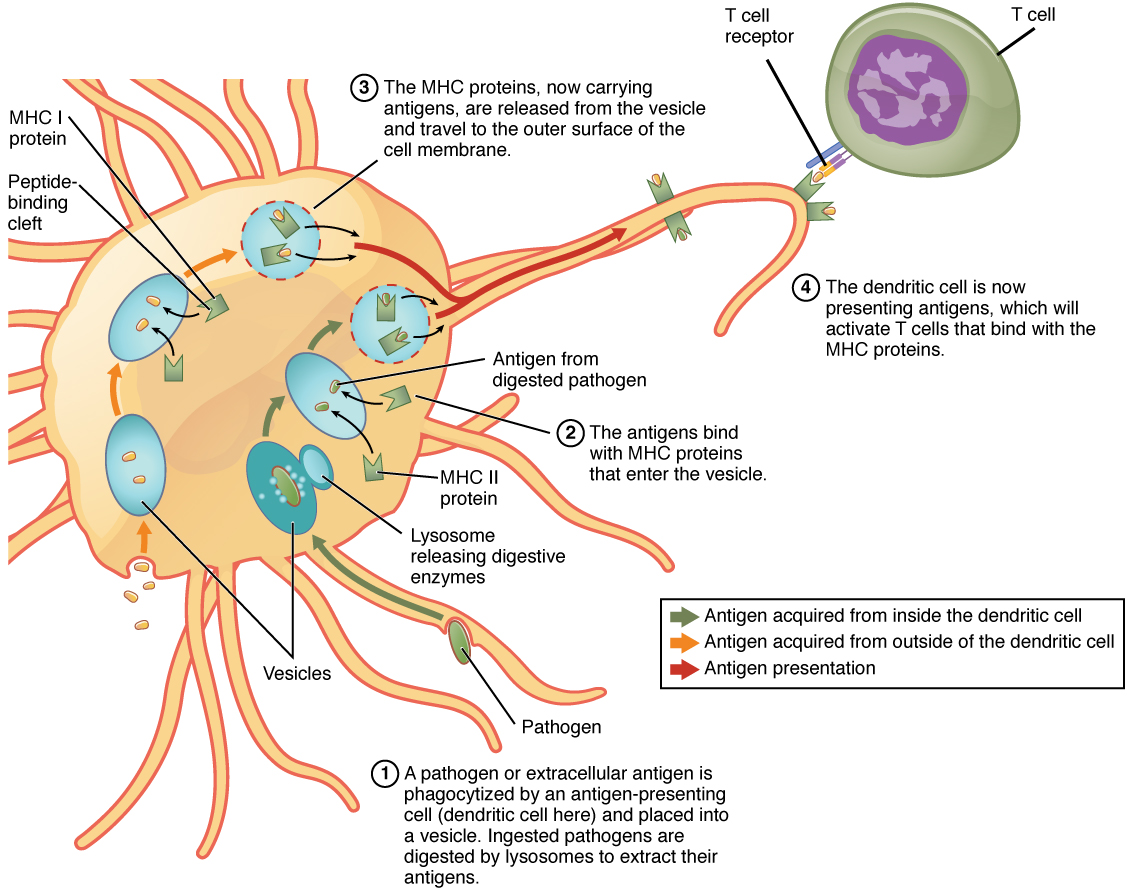 Figure 21.17 Antigen Processing and Presentation
Figure 21.17 Antigen Processing and Presentation
Two distinct types of MHC molecules, MHC class I and MHC class II, play roles in antigen presentation. Although produced from different genes, they both have similar functions. They bring processed antigen to the surface of the cell via a transport vesicle and present the antigen to the T cell and its receptor. Antigens from different classes of pathogens, however, use different MHC classes and take different routes through the cell to get to the surface for presentation. The basic mechanism, though, is the same. Antigens are processed by digestion, are brought into the endomembrane system of the cell, and then are expressed on the surface of the antigen-presenting cell for antigen recognition by a T cell. Intracellular antigens are typical of viruses, which replicate inside the cell, and certain other intracellular parasites and bacteria. These antigens are processed in the cytosol by an enzyme complex known as the proteasome and are then brought into the endoplasmic reticulum by the transporter associated with antigen processing (TAP) system, where they interact with class I MHC molecules and are eventually transported to the cell surface by a transport vesicle.
Extracellular antigens, characteristic of many bacteria, parasites, and fungi that do not replicate inside the cell’s cytoplasm, are brought into the endomembrane system of the cell by receptor-mediated endocytosis. The resulting vesicle fuses with vesicles from the Golgi complex, which contain pre-formed MHC class II molecules. After fusion of these two vesicles and the association of antigen and MHC, the new vesicle makes its way to the cell surface.
Professional Antigen-presenting Cells
Many cell types express class I molecules for the presentation of intracellular antigens. These MHC molecules may then stimulate a cytotoxic T cell immune response, eventually destroying the cell and the pathogen within. This is especially important when it comes to the most common class of intracellular pathogens, the virus. Viruses infect nearly every tissue of the body, so all these tissues must necessarily be able to express class I MHC or no T cell response can be made.
On the other hand, class II MHC molecules are expressed only on the cells of the immune system, specifically cells that affect other arms of the immune response. Thus, these cells are called “professional” antigen-presenting cells to distinguish them from those that bear class I MHC. The three types of professional antigen presenters are macrophages, dendritic cells, and B cells (Table 21.4).
Macrophages stimulate T cells to release cytokines that enhance phagocytosis. Dendritic cells also kill pathogens by phagocytosis (see Figure 21.17), but their major function is to bring antigens to regional draining lymph nodes. The lymph nodes are the locations in which most T cell responses against pathogens of the interstitial tissues are mounted. Macrophages are found in the skin and in the lining of mucosal surfaces, such as the nasopharynx, stomach, lungs, and intestines. B cells may also present antigens to T cells, which are necessary for certain types of antibody responses, to be covered later in this chapter.
Classes of Antigen-presenting Cells
MHC |
Cell type |
Phagocytic? |
Function |
Class I |
Many |
No |
Stimulates cytotoxic T cell immune response |
Class II |
Macrophage |
Yes |
Stimulates phagocytosis and presentation at primary infection site |
Class II |
Dendritic |
Yes, in tissues |
Brings antigens to regional lymph nodes |
Class II |
B cell |
Yes, internalizes surface Ig and antigen |
Stimulates antibody secretion by B cells |
Table 21.4
T Cell Development and Differentiation
The process of eliminating T cells that might attack the cells of one’s own body is referred to as T cell tolerance. While thymocytes are in the cortex of the thymus, they are referred to as “double negatives,” meaning that they do not bear the CD4 or CD8 molecules that you can use to follow their pathways of differentiation (Figure 21.18). In the cortex of the thymus, they are exposed to cortical epithelial cells. In a process known as positive selection, double-negative thymocytes bind to the MHC molecules they observe on the thymic epithelia, and the MHC molecules of “self” are selected. This mechanism kills many thymocytes during T cell differentiation. In fact, only two percent of the thymocytes that enter the thymus leave it as mature, functional T cells.
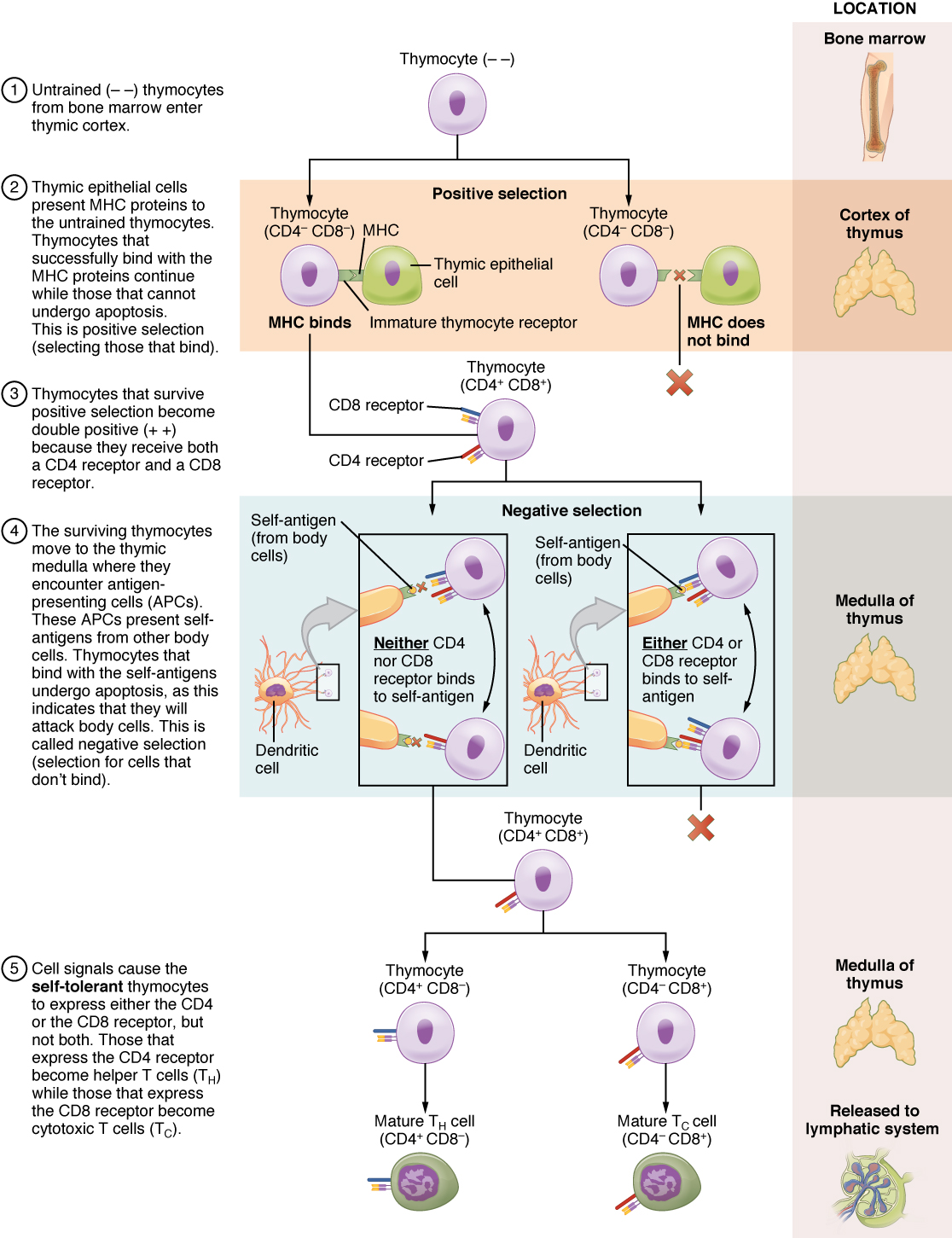 Figure 21.18 Differentiation of T Cells within the Thymus Immature T-cells, called thymocytes, enter the thymus and go through a series of developmental stages that ensures both function and tolerance before they leave and become functional components of the adaptive immune response.
Figure 21.18 Differentiation of T Cells within the Thymus Immature T-cells, called thymocytes, enter the thymus and go through a series of developmental stages that ensures both function and tolerance before they leave and become functional components of the adaptive immune response.
Later, the cells become double positives that express both CD4 and CD8 markers and move from the cortex to the junction between the cortex and medulla. It is here that negative selection takes place. In negative selection, self-antigens are brought into the thymus from other parts of the body by professional antigen-presenting cells. The T cells that bind to these self-antigens are selected for negatively and are killed by apoptosis. In summary, the only T cells left are those that can bind to MHC molecules of the body with foreign antigens presented on their binding clefts, preventing an attack on one’s own body tissues, at least under normal circumstances. Tolerance can be broken, however, by the development of an autoimmune response, to be discussed later in this chapter.
The cells that leave the thymus become single positives, expressing either CD4 or CD8, but not both (see Figure 21.18). The CD4+ T cells will bind to class II MHC and the CD8+ cells will bind to class I MHC. The discussion that follows explains the functions of these molecules and how they can be used to differentiate between the different T cell functional types.
Mechanisms of T Cell-mediated Immune Responses
Mature T cells become activated by recognizing processed foreign antigen in association with a self-MHC molecule and begin dividing rapidly by mitosis. This proliferation of T cells is called clonal expansion and is necessary to make the immune response strong enough to effectively control a pathogen. How does the body select only those T cells that are needed against a specific pathogen? Again, the specificity of a T cell is based on the amino acid sequence and the three-dimensional shape of the antigen-binding site formed by the variable regions of the two chains of the T cell receptor (Figure 21.19). Clonal selection is the process of antigen binding only to those T cells that have receptors specific to that antigen. Each T cell that is activated has a specific receptor “hard-wired” into its DNA, and all of its progeny will have identical DNA and T cell receptors, forming clones of the original T cell.
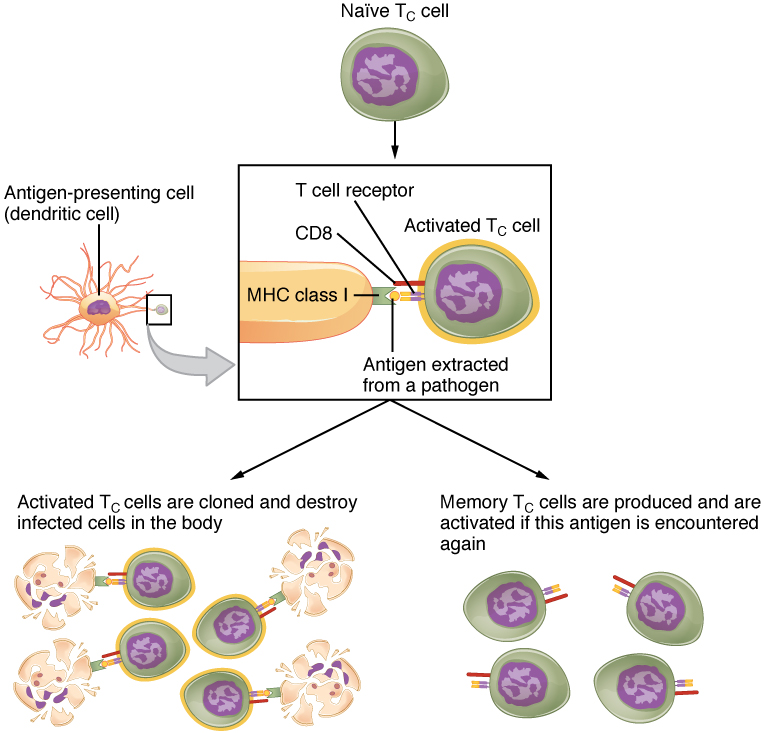 Figure 21.19 Clonal Selection and Expansion of T Lymphocytes Stem cells differentiate into T cells with specific receptors, called clones. The clones with receptors specific for antigens on the pathogen are selected for and expanded.
Figure 21.19 Clonal Selection and Expansion of T Lymphocytes Stem cells differentiate into T cells with specific receptors, called clones. The clones with receptors specific for antigens on the pathogen are selected for and expanded.
Clonal Selection and Expansion
The clonal selection theory was proposed by Frank Burnet in the 1950s. However, the term clonal selection is not a complete description of the theory, as clonal expansion goes hand in glove with the selection process. The main tenet of the theory is that a typical individual has a multitude (1011) of different types of T cell clones based on their receptors. In this use, a clone is a group of lymphocytes that share the same antigen receptor. Each clone is necessarily present in the body in low numbers. Otherwise, the body would not have room for lymphocytes with so many specificities.
Only those clones of lymphocytes whose receptors are activated by the antigen are stimulated to proliferate. Keep in mind that most antigens have multiple antigenic determinants, so a T cell response to a typical antigen involves a polyclonal response. A polyclonal response is the stimulation of multiple T cell clones. Once activated, the selected clones increase in number and make many copies of each cell type, each clone with its unique receptor. By the time this process is complete, the body will have large numbers of specific lymphocytes available to fight the infection (see Figure 21.19).
The Cellular Basis of Immunological Memory
As already discussed, one of the major features of an adaptive immune response is the development of immunological memory.
During a primary adaptive immune response, both memory T cells and effector T cells are generated. Memory T cells are long-lived and can even persist for a lifetime. Memory cells are primed to act rapidly. Thus, any subsequent exposure to the pathogen will elicit a very rapid T cell response. This rapid, secondary adaptive response generates large numbers of effector T cells so fast that the pathogen is often overwhelmed before it can cause any symptoms of disease. This is what is meant by immunity to a disease. The same pattern of primary and secondary immune responses occurs in B cells and the antibody response, as will be discussed later in the chapter.
T Cell Types and their Functions
In the discussion of T cell development, you saw that mature T cells express either the CD4 marker or the CD8 marker, but not both. These markers are cell adhesion molecules that keep the T cell in close contact with the antigen-presenting cell by directly binding to the MHC molecule (to a different part of the molecule than does the antigen). Thus, T cells and antigen-presenting cells are held together in two ways: by CD4 or CD8 attaching to MHC and by the T cell receptor binding to antigen (Figure 21.20).
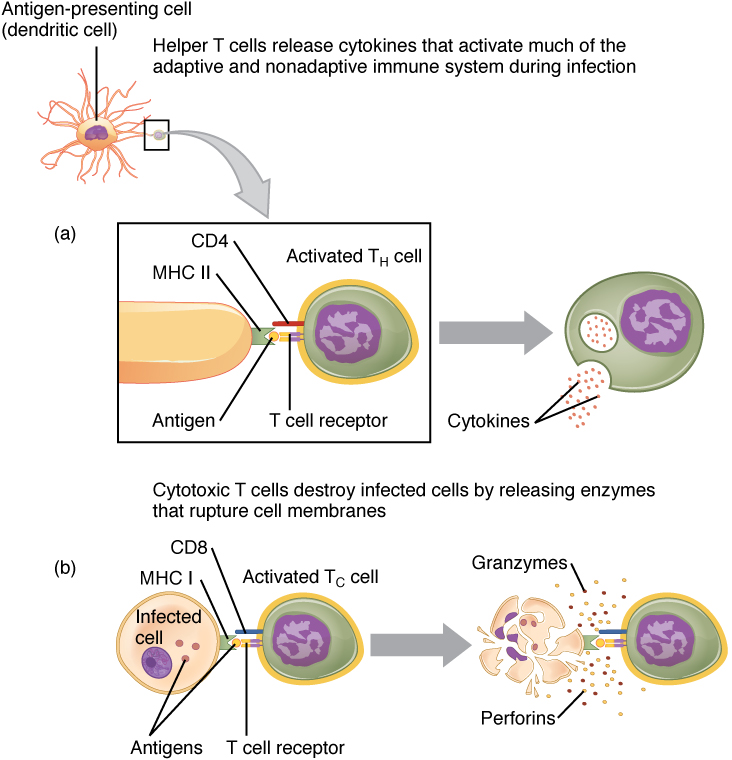 Figure 21.20 Pathogen Presentation (a) CD4 is associated with helper and regulatory T cells. An extracellular pathogen is processed and presented in the binding cleft of a class II MHC molecule, and this interaction is strengthened by the CD4 molecule. (b) CD8 is associated with cytotoxic T cells. An intracellular pathogen is presented by a class I MHC molecule, and CD8 interacts with it.
Figure 21.20 Pathogen Presentation (a) CD4 is associated with helper and regulatory T cells. An extracellular pathogen is processed and presented in the binding cleft of a class II MHC molecule, and this interaction is strengthened by the CD4 molecule. (b) CD8 is associated with cytotoxic T cells. An intracellular pathogen is presented by a class I MHC molecule, and CD8 interacts with it.
Although the correlation is not 100 percent, CD4-bearing T cells are associated with helper functions and CD8-bearing T cells are associated with cytotoxicity. These functional distinctions based on CD4 and CD8 markers are useful in defining the function of each type.
Helper T Cells and their Cytokines
Helper T cells (Th), bearing the CD4 molecule, function by secreting cytokines that act to enhance other immune responses. There are two classes of Th cells, and they act on different components of the immune response. These cells are not distinguished by their surface molecules but by the characteristic set of cytokines they secrete (Table 21.5).
Th1 cells are a type of helper T cell that secretes cytokines that regulate the immunological activity and development of a variety of cells, including macrophages and other types of T cells.
Th2 cells, on the other hand, are cytokine-secreting cells that act on B cells to drive their differentiation into plasma cells that make antibody. In fact, T cell help is required for antibody responses to most protein antigens, and these are called T cell-dependent antigens.
Cytotoxic T cells
Cytotoxic T cells (Tc) are T cells that kill target cells by inducing apoptosis using the same mechanism as NK cells. They either express Fas ligand, which binds to the fas molecule on the target cell, or act by using perforins and granzymes contained in their cytoplasmic granules. As was discussed earlier with NK cells, killing a virally infected cell before the virus can complete its replication cycle results in the production of no infectious particles. As more Tc cells are developed during an immune response, they overwhelm the ability of the virus to cause disease. In addition, each Tc cell can kill more than one target cell, making them especially effective. Tc cells are so important in the antiviral immune response that some speculate that this was the main reason the adaptive immune response evolved in the first place.
Regulatory T Cells
Regulatory T cells (Treg), or suppressor T cells, are the most recently discovered of the types listed here, so less is understood about them. In addition to CD4, they bear the molecules CD25 and FOXP3. Exactly how they function is still under investigation, but it is known that they suppress other T cell immune responses. This is an important feature of the immune response, because if clonal expansion during immune responses were allowed to continue uncontrolled, these responses could lead to autoimmune diseases and other medical issues.
Not only do T cells directly destroy pathogens, but they regulate nearly all other types of the adaptive immune response as well, as evidenced by the functions of the T cell types, their surface markers, the cells they work on, and the types of pathogens they work against (see Table 21.5).
Functions of T Cell Types and Their Cytokines
T cell |
Main target |
Function |
Pathogen |
Surface marker |
MHC |
Cytokines or mediators |
Tc |
Infected cells |
Cytotoxicity |
Intracellular |
CD8 |
Class I |
Perforins, granzymes, and fas ligand |
Th1 |
Macrophage |
Helper inducer |
Extracellular |
CD4 |
Class II |
Interferon-γ and TGF-β |
Th2 |
B cell |
Helper inducer |
Extracellular |
CD4 |
Class II |
IL-4, IL-6, IL-10, and others |
Treg |
Th cell |
Suppressor |
None |
CD4, CD25 |
? |
TGF-β and IL-10 |
Table 21.5

