7 Myofascial Injury
7.1 Myogenous Pain Assessment and Treatment
Claudia’s persistent complaint of pain along with her physical assessment of cervical range of motion, muscle length, and trigger point assessment indicated a possible myofascial injury (also called soft tissue injury in layman terms, a common element of WAD II). Emerging scientific evidence supports the presence of structural (pathophysiological) changes in cervical musculature1,2 and neurophysiological alterations in cervical and scapulothoracic muscular movement,3-9 synergy,8,10 and functional control.11 Motor control alterations are multivariate. Muscular dysfunction can also be influenced by other central nervous system factors such as reflexes mediated through eye movement resulting in postural control changes12,13 in the neck or mediated through cognitive or affective changes.14,15 Muscle dysfunctions in those with a persistent whiplash are identified clinically with signs and symptoms of neck muscle imbalance, postural sway, and increased resting tone. Patients with chronic WAD are known to have clinical changes in cervical muscle function or activity, with abnormal range of motion, in addition to alterations in postural control or balance.16 Clinical measurement of muscular and myofascial variables are therefore used to document the presence of myogenous or myofascial dysfunction. Diagnostic imaging often fails to detect the structural pathology related to the cause of pain in WAD. That being said, emerging imaging techniques (functional MRI, ultrasound) may be used to differentiate the underlying muscular elements. There are radiological, ultrasonography, and MRI studies that demonstrate evidence of upper cervical ligamentous disruption, altered segmental motion, and muscular degeneration.17
Claudia was referred to a specialist multidisciplinary pain clinic for additional assessment and treatment.
7.2 Functional Anatomy of Myofascial Structures in the Neck and Scapulothoracic Region
A brief description of the cervical myofascial anatomy has been provided in Chapter 3.7. At the end of this section, the functional anatomical details are discussed in the form of a quiz (cervical muscles, Quiz 7-1; cervico-scapulo-thoracic muscles, Quiz 7-2), with a summary.
Neck motion can be divided into rotation (looking side to side), lateral flexion (ear to shoulder), flexion (chin to sternum), and extension (looking up). The deep muscle layers of the cervical spine have a unique capacity to contribute to control of intersegmental motion.18,19 The relationship between the deep and superficial neck muscles has been investigated to improve the understanding of the complexity of neck muscle function20 and change due to the presence of pain. Knowing the motions associated with each muscle will aid in evaluation and treatment planning. Details are presented in Table 7-1, Table 7-2, Table 7-3, and Table 7-4, as well as Figure 7-1 and Figure 7-2.
TABLE 7.1 Pairing of shoulder girdle and shoulder joint movements
| Shoulder joint | Shoulder girdle |
| Abduction | Upward rotation |
| Adduction | Downward rotation |
| Flexion | Elevation/upward rotation |
| Extension | Depression/downward rotation |
| Internal rotation | Abduction (protraction) |
| External rotation | Adduction (retraction) |
| Horizontal abduction | Adduction (retraction) |
| Horizontal adduction | Abduction (protraction) |
TABLE 7.2 Summary of functional anatomy of cervical muscles
| Flexors | Longus colli, longus capitis, rectus capitis anterior (rectus capitus anterior, medius, rectus capitus lateralis), and infrahyoids (superficial group: omohyoid, sternohyoid, deep group: thyroid, sternothyroid, cricothyroid). Note: the suprahyoid group relate primarily to tongue (mylohyoid, stylohyoid) and TMJ (digastric posterior and anterior belly) function. See Image 7-1 |
| Extensors | Superficial group: trapezius Transversocostal group: splenius capitis, splenius cervicis, iliocostalis cervicis, longissimus cervicis Transversospinal group: semispinalis capitis, semispinalis cervicis, multifidus; Deep group: suboccipitals (rectus capitus, major, and rectus capitus minor). See Image 7-2 |
| Rotators | Splenius capitis, sternocleidomastoid, levator scapula, suboccipitals Left rotation: right superior oblique, left inferior oblique Right rotation: left superior oblique, right inferior oblique. See Image 7-3 |
| Lateral flexors | Scalenes (anterior, medius, posterior) Right side flexion (suboccipitals): right superior oblique, right rectus capitus major, right rectus capitus minor Left side flexion (suboccipitals): left superior oblique, left rectus capitus major, left rectus capitus minor. See Image 7-4 |
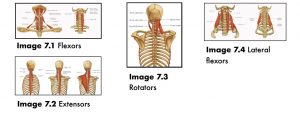
TABLE 7.3 Summary of functional anatomy of scapulothoracic muscles
| Scapular movement | Prime movers |
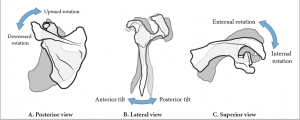
| Upward rotation of the scapula | a. upper fibers of trapezius b. lower fibers of trapezius c. serratus anterior |
| Downward rotation of the scapula | a. rhomboid b. levator scapulae c. latissimus dorsi d. pectoralis minor |
| Anterior tipping of the scapula | a. superior fibers of the serratus anterior b. pectoralis minor |
| Posterior tipping of the scapula | a. lower fibers of trapezius |
| Scapular protraction | a. pectoralis minor b. serratus anterior |
| Scapular retraction | a. middle fibers of trapezius b. rhomboids |
| Scapular elevation | a. levator scapulae b. upper fibers of trapezius |
| Scapular depression | a. lower fibers of trapezius b. pectoralis minor c. lower fibers of serratus anterior |
TABLE 7.4 Optimal posture (cervical neutral/axial extension) can be impaired with key muscles demonstrating activity and inhibition
| Hyperactive | Inhibited | |
| Dorsal neck muscles | Mid and upper trapezius, levator scapulae, suboccipital muscles | Lower trapezius, supra- and infraspinatus, deltoid, deep lower neck extensors |
| Anterior neck muscle | Pectoralis, scalene (anterior, middle, posterior), sternocleidomastoid | Deep neck flexors, longus colli and capitus, serratus anterior |
Quiz 7.1: Cervical Muscles
1. The longus colli and infrahyoids are the primary muscles that are used for neck flexion. Choose from the following:
A. True
B. False
2. The suboccipitals are involved in neck rotation. For rotational movement towards right, which muscles are primarily involved?
A. Rectus capitis major
B. Right inferior oblique
C. Left superior oblique
D. Right superior oblique
E. B and C
F. A and D
3. Deep anterior neck muscles are often noted to have reduced activation after whiplash injury. Which muscles does this include?
A. Longus colli
B. Sternocleidomastoid
C. Rectus capitis anterior
D. Infrahyoid
E. A, C, and D
F. All of the above
4. The whiplash patient is noted to have restriction of right-side flexion. Which muscle(s) are primarily shortened
A. Scalene anterior
B. Scalene medius
C. Scalene posterior
D. Sternocleidomastoid
E. A and D
F. A, B, and C
5. Neck extensors include three layers of muscles including: the superficial layer – trapezius; the middle layer – semispinalis capitis and splenius capitis; as well as the deep layer – suboccipitals
A. True
B. False
1. A; 2. E; 3. E; 4. F; 5. B
Quiz 7.2: Cervico-Scapulo-Thoracic Muscles
1. Upward rotation (Figure 7-1 and 7-2; Table 7-2 and 7-3) of the scapula includes which of the following scapulothoracic muscle groups?
A. Upper fibers of trapezius
B. Lower fibers of trapezius
C. Serratus anterior
D. All of the above
E. Upper fibres of trapezius and serrates anterior
2. Downward rotation (Figure 7-1) of the scapula requires which of the following muscles to be the prime movers?
A. Rhomboid
B. Levator scapula
C. Latissimus dorsi
D. Pectoralis major
E. All of the above
F. A, B, and C
3. Optimal posture can be impaired due to scapulothoracic and neck muscle weakness. Which of the following muscles are commonly inhibited during resting forward head postures?
A. Dorsal neck muscles: mid and upper trapezius, levator scapulae, suboccipitals
B. Anterior neck muscles: pectoralis (major/minor), scalene, sternocleidomastoid, longus colli
C. All of the above
D. None of the above
4. What is a cause of serratus anterior muscle weakness?
A. Short rhomboid and levator scapula shortening
B. Short lower fibers and upper fibers of trapezius shortening
C. Pectoralis minor shortening
D. A and C
1. D; 2. F; 3. C; 4. A
7.3 Clinical Features of Injury and Physical Testing for Muscle Pathology
Testing for Altered Movements and Stiffness
7.3.1. Weak Deep Neck Flexion and Altered Motor Control
In people with persisting neck pain after whiplash, morphological changes in neck muscles manifest as altered neuromuscular control patterns in the cervical region. Morphological changes in the deep anterior neck muscles (longus capitis/colli) include greater muscle fatty infiltrate and cross sectional area compared with healthy controls.21 The emerging functional consequences include both spatial and temporal behavioral changes in cervical muscles. Spatial changes include a substitution pattern of cervical muscle activation between the deep and superficial cervical flexors.22-25 As the deep neck muscles (longus colli and longus capitis) become weaker, a pattern of increased activation of superficial muscles (sternocleidomastoid) emerges.23 There is also evidence of temporal behavior changes in cervical muscles.26-28 Delayed recruitment onset of the deep cervical flexors, cervical extensors, and sternocleidomastoid muscles is noted relative to anterior deltoid activation during rapid arm movement. Normally, cervical flexor and extensor muscles should display rapid feed-forward co-activation within approximately 50 ms of the onset of deltoid activation during rapid arm movement. Additionally, the linear relationship (deformation [elongation and shortening] and deformation rate) between anterior neck muscles (the longus capitis, longus colli and sternocleidomastoid) is weak or missing in those with whiplash.23 The variability or interplay between anterior neck muscles is less than in normal control subjects.23 Thus, impaired neuromuscular control of the cervical spine exists26,27,29 and is associated with neck pain.30 Persisting deficits in muscle control and disturbed motor patterning results in reduced strength and endurance25,26,31,32 in those suffering ongoing pain, those with high pain/disability as well as in those who did not report full recovery at up to two years post whiplash injury.5 Three craniocervical flexion tests were developed to help clinicians measure these impairments.
Cranio-cervical Flexion Test
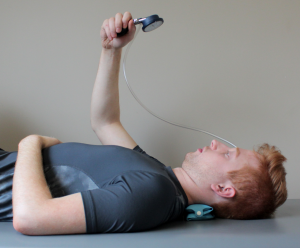
The Cranio-cervical Flexion Test33 is performed with the patient in supine position. Start position: Supine crook lying head in neutral position, the forehead is in line with chin. (towels may be placed under the occiput to achieve this). A pressure biofeedback cuff is placed under the neck, filled to 20 mmHg and air distribution calibrated (Figure 7-3). Craniocervical flexion, a gentle upper cervical nod, is performed in five increments (22, 24, 26, 28, and 30 mmHg). Each increment is held isometrically for 10 seconds. There is a 10-second rest period between increments. The clinician palpates the sternocleidomastoid for unwanted activation, monitors the pressure level (a decrease of 20% is abnormal), and monitors for substitution strategies (i.e., jerky movement) or superficial cervical muscle use (platysma, hyoid muscle, or sternocleidomastoid).
Normal value: 26–30 mmHg maintained for 10 seconds without substitution strategy.
Reliability: good ICC 0.81, performance index ICC 0.93.33
Cranio-cervical Flexion Endurance Test
The Cranio-cervical Flexion Endurance Test33 is used to test isometric endurance.
Start position: Same as the previous test but instead of performing one repetition per 22, 23, 24, 26 mmHg it is 10 repetitions with 10-second hold at each increment; 10-second rest between increments.
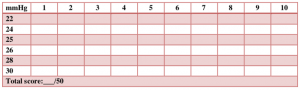
Note each repetition on chart (Figure 7-4); there are 10 per increment, sum each row to attain the total score out of 50.33
Normal values: 26–30 mmHg maintained for 10 seconds without substitution strategy and total score >30/50
Reliability: good ICC 0.81, performance index ICC 0.93.33
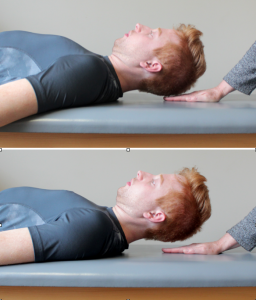
Deep Neck Flexor Muscle Endurance Test34,35
Start position: Supine crook lying with head in neutral (the forehead is in line with the chin). A mark is placed in the anterolateral skin fold. Isometric cranio-cervical flexion is maintained as the head is lifted approximately 2.5 cm (1 inch). The clinician monitors the skin fold mark, places hand on plinth just under the occiput, and provides verbal cues “tuck your chin” and “hold your head up” (Figure 7-5). The clinician also monitors for substitution strategy; when skin folds separate and mark is observed, or the patient’s occiput touches the therapist’s hand for more than 1 second the test ends.
Normal values: Men: 38.9 seconds, women: 29.4 seconds.36 Those with neck pain average 21.4 seconds.
Reliability: Good without neck pain: ICC 0.67–0.91, SEM 8.0–15.3 seconds; with neck pain: ICC 0.67, SEM 11.5 seconds.34,35
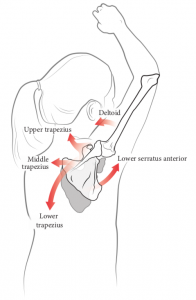
7.3.2. Altered Scapulothoracic Motor Control
In chronic WAD, there are altered patterns of muscle motor control and recruitment in the scapulothoracic region.37,38 During arm elevation tasks of the affected arm, there is a delayed activation in the serratus anterior muscle39 and increased activity of upper fibers of trapezius.40 During the lowering phase of an overhead reach and grasp task, there is reduced activation of upper fibers of trapezius, lower fibers of trapezius, anterior deltoid, and serratus anterior.41 The resulting scapular dyskinesis is most prominent during the lowering phase. Impaired eccentric control of the scapula will cause altered scapular kinematics and shoulder pain.41 Additionally, cervical kinematic impairments strongly correlate with self-reported pain, disability, dizziness, and balance deficits.28 Spinal kinematics such as increased thoracic extension and contralateral side flexion occurs in people with neck pain.42 Modification of spinal movement to complete a functional task such as reach and grasp was found to be the main cause of impaired kinematics.42
Tests for scapulothoracic strength and upper quadrant function were developed to help clinicians measure impairments.
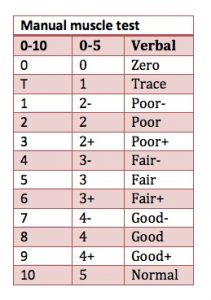
Scapulothoracic Manual Muscle Testing43
Scapulothoracic manual muscle testing is demonstrated here: (https://youtu.be/oFI32NmCKts). It tests the upper, middle, and lower fibers of trapezius, rhomboid, serratus anterior, pectoralis minor, and pectoralis major.
Reliability: Kendall’s manual muscle testing 0–5 scale has limited reliability. Kendall’s manual muscle testing 0–10 scale has excellent reliability (r=0.90–0.95) (Figure 7-6); good internal consistency (ICC 0.70–0.75), and moderate construct validity (r=0.47–0.70) validity.44,45
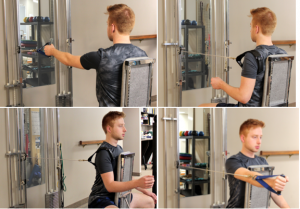
Scapular muscles maximal isometric force measurement46
Hand-held dynamometers have good reliability, but depend on the strength of the tester. The Isobex® stationary tension dynamometer is used to measure the maximal isometric force (kg).46 Key scapular stabilizing muscles for protraction (serratus anterior, upper trapezius, and pectoralis minor muscles) and retraction (middle trapezius and rhomboid muscles) movements, with and without the involvement of the glenohumeral joint and rotator cuff muscles (Figure 7-7).
Reliability: Intrasession reliability ICC 0.95–0.98; intersession reliability ICC 0.94–0.96. SEM (95% CI) were narrow.
FitHansa Protocol
The Functional Impairment Test: Head, and Neck/Shoulder/Arm (FitHansa) protocol tests the endurance of the cervico-scapulothoracic region47,48 The FitHansa protocol is designed to measure the functional performance of the shoulder region at three levels (Figure 7-8):

Test 1 – “Waist-Up”
Test 2 – “Eye-Down”
Test 3 – “Overhead Work”
Scoring: The times are measured using a stopwatch.
Test 1 (Waist-Up)/300 sec × 100%
Test 2 (Eye-Down)/300 sec × 100%
Test 3 (Overhead Work)/300 sec × 100%
Total Score = Mean of Test 1, Test 2 and Test 3
Reliability: Test-retest reliability ICC 0.89–0.97 in the patient group and 0.79–0.91 in the control group; high correlation with Disabilities of the Arm, Shoulder and Hand (DASH) and the Shoulder Pain and Disability Index (SPADI), and moderate correlations with shoulder range of motion and muscle strength.47
Intra- and inter-ICCs 0.88–0.89 in the control group and 0.78– 0.85 in the WAD II group.48
7.3.3 Weak Deep Neck Extensors and Reduced Deep Extensor Activation
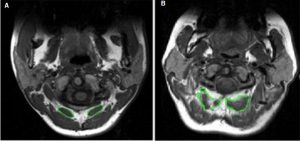
Marked morphological changes to cervical spine extensor muscles in people with chronic whiplash also occur (Figure 7-9). Using MRI, Elliott et al.49,50 demonstrated the presence of fatty infiltrate in both deep and superficial cervical extensor and flexor muscles compared with an asymptomatic control group. These infiltrates develop within one to three months following injury in those with more severe levels of pain and disability and appears to be highest in the deeper muscles (rectus capitis minor/major and multifidus).49 Using ultrasound, a cross-sectional area difference in semispinalis capitis was noted between those with intermittent headache after whiplash contrasted against controls.22,25 O’Leary et al.51 determined changes in spatial relations between extensors and reduced activity in deep extensors semispinalis cervicis, splenius cervicis, and multifidus on muscle functional MRI in those with persisting neck pain. Specific muscles (multifidus, splenius cervicis) had significantly lower activation at both the C5-C6 and C7-T1 levels when compared with the control group performing the craniocervical neutral neck extension exercise. As already noted, the temporal behaviour changes exist; that is, the neuromuscular control pattern of splenius capitus, multifidus, and cervical extensor muscles should demonstrate feedforward activation (activation within 50 ms of deltoid onset) during rapid arm movements in all directions. The sequence and magnitude of neck muscle activation display directional specificity; however, the neck flexor and extensor muscles should display co-activation during all perturbations.26-28 Again, tests of neck extensor strength and endurance were developed to measure elements of these impairments.
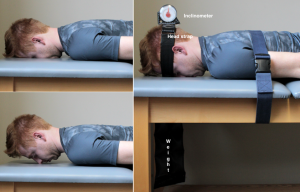
Neck Extensor Endurance Test52,53
The neck extensor endurance test is demonstrated in Figure 7-10.
Test 1: Hold in neutral extension
Test 2: Weighted hold in neutral extension
Subject position: Prone lying with head supported by plinth headrest and body stabilized by a belt at the thoracic spine at T6. An inclinometer is attached to the head strap over the occiput. The inclinometer on the subject’s head is used to monitor the head position during the test. A 2 kg weight is suspended from the subject’s head. Their head is positioned in neutral sagittal plane position and the test is initiated when the examiner removes the support from the subject’s head. The subject is required to hold the cervical spine horizontal with the chin retracted. The test is terminated if the weight returns to the floor or if the neck position changes by more than 5° from the horizontal, as measured by the inclinometer. The holding time is measured in seconds.
Score: Normal, 228 seconds; neck pain, 165 seconds; SEM, 25.9 seconds. The minimum change required to represent real change is 71.3 seconds for the neck extensor test.
Reliability and validity: Unclear.
7.3.4 Altered Proprioception Acuity
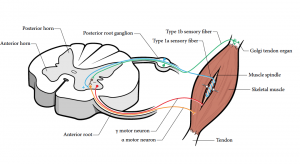
Persisting nociception affects not only motor but also proprioceptive pathways.54 Somatosensory processing including proprioception is influenced by tonic muscle nociception and modulation of muscle spindle afferent activity. This in turn impairs the efficiency and precision of movements. Sympathetic activation impairs or decreases proprioceptive acuity. Muscle spindle afferents or Golgi tendon organs can impair the ability of the central nervous system to use proprioceptive information. See Figure 7-11 to review these pathways. Motor control retraining is thus prevented by nociception-induced motor inhibition. Two testing procedures were developed to measure proprioceptive acuity in the clinical setting: 1) mean error of proprioception; and 2) neck motion kinematics.55,56
Proprioception – Mean Error Test Protocol56
Protocol Steps: Patient seated 80 cm from target, torso stabilized in seated position. ‘Head neutral’ is attained and the laser target is centered. Six repetitions of left rotation is performed with eyes closed, reset to head neutral between repetitions. Measure each of six repetitions, add them together and divide by six for horizontal mean error (Figure 7-12)
Scoring: Mean error of measure:__+__+__+__+__+__ =__ ÷6 Horizontal = _____cm
Validity: The test set of this tool has been established. The validation set and reliability have not been established.

Neck Motion Kinematics using Virtual Reality
The interactive neck virtual reality tool assesses neck motion kinematics (Figure 7-13). Neck motion kinematics has a published protocol.55
Reliability: 1) Peak and mean velocity. 2) Minimal detectable change for peak velocity ranged from 41 to 53 degrees/second, noting that a velocity larger than this is a true change. Minimal detectable change for mean velocity was from 20 to 25 degrees/second, indicating a change in cervical motion velocity greater than approximately 20 should be regarded as indicative of a true change.

7.3.5 Clinical Tests
While the specific tissue causing a patient’s neck pain may remain clinically elusive and may or may not be associated with degenerative changes or pathology identified during diagnostic imaging/tests, the clinician should assess for impaired muscle function, connective tissue, and neural elements associated with the identified pathological region. We reviewed testing of myofascial connective tissue elements using trigger point palpation and algometry. Palpation of myofascial trigger points for hyperalgesia is a subjective test but by using algometry the pain pressure threshold for selected trigger points can be a semi-objective test.57,58
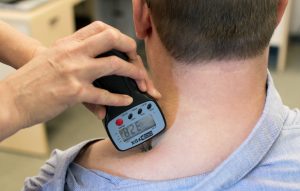
Pain Pressure Threshold Test
Protocol: Measurement of mechanical hyperalgesia using pressure algometry has been discussed in Chapter 6-3 and is shown in Figure 7-14.
Scoring: An Android-based Smartphone app for interpreting pain pressure threshold scores can be downloaded for free from http://www.pirlresearch.com/clinician-resources#PPDT_app 59-61
Reliability and Validity: Previously discussed in Chapter 6-3.
Myofascial Assessment
Protocol for palpation of myofascial trigger points: 1) Locate, then palpate. 2) Press on the site using two pounds of pressure for 1 second. 3) Apply the prescribed amount of pressure on the site without moving the fingers sideways or rubbing the area. 4) Cover the full area of the muscle within each defined region. 5) Ask about related pain:
- Presence of pain: is it painful when I press here?
- Familiar pain: is the pain similar to yours?
- Headache replication: Referral of pain – when I press in this area does the cause pain anywhere else? Is it familiar pain?
- Replication of referral: is it a familiar pattern?
Scoring: Can include a count of positive trigger points and a scoring of pain sensitivity on numeric rating scale 0–10.
Reliability and validity estimates: Vary widely for each diagnostic sign, for each muscle, and across each study. Reliability estimates are higher for subjective signs such as tenderness (κ range, 0.22–1.0) and pain reproduction (κ range, 0.57–1.00), and lower for objective signs such as the taut band (κ range, −0.08–0.75), and local twitch response (κ range, −0.05–0.57).61,62
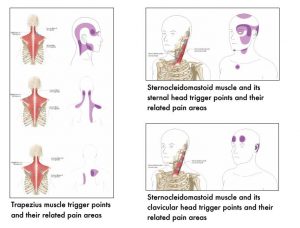
The following images show muscle-specific trigger points and their related pain areas.
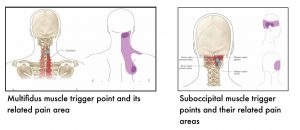
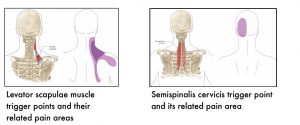
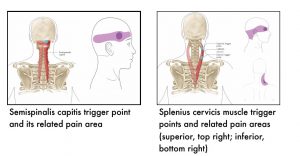
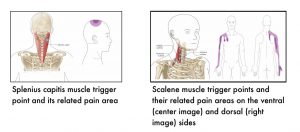
7.4 References
- Elliott JM. Are there implications for morphological changes in neck muscles after whiplash injury? Spine (Phila Pa 1976) 2011;36(25 Suppl):S205-10.
- Elliott JM, Pedler AR, Jull GA, Van Wyk L, Galloway GG, O’Leary SP. Differential changes in muscle composition exist in traumatic and nontraumatic neck pain. Spine (Phila Pa 1976) 2014;39:39-47.
- Cagnie B, Dirks R, Schouten M, Parlevliet T, Cambier D, Danneels L. Functional reorganization of cervical flexor activity because of induced muscle pain evaluated by muscle functional magnetic resonance imaging. Man Ther 2011;16(5):470-5.
- Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain 2011;152(3 Suppl):S90-8.
- Lindstrøm R, Schomacher J, Farina D, Rechter L, Falla D. Association between neck muscle coactivation, pain, and strength in women with neck pain. Man Ther 2011;16:80-6.
- Madeleine P, Mathiassen SE, Arendt-Nielsen L. Changes in the degree of motor variability associated with experimental and chronic neck-shoulder pain during a standardised repetitive arm movement. Exp Brain Res 2008;185:689-98.
- Moseley GL, Hodges PW. Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: a risk factor for chronic trouble? Behav Neurosci 2006;120:474-6.
- Muceli S, Falla D, Farina D. Reorganization of muscle synergies during multidirectional reaching in the horizontal plane with experimental muscle pain. J Neurophysiol 2014;111:1615-30.
- Sullivan MJ, Lariviere C, Simmonds M. Activity-related summation of pain and functional disability in patients with whiplash injuries. Pain 2010;151:440-6.
- Gizzi L, Muceli S, Petzke F, Falla D. Experimental muscle pain impairs the synergistic modular control of neck muscles. PLoS One 2015;10:e0137844.
- Falla D, Farina D. Neural and muscular factors associated with motor impairment in neck pain. Curr Rheumatol Rep 2007;9:497-502.
- Bexander CS, Hodges PW. Cervico-ocular coordination during neck rotation is distorted in people with whiplash-associated disorders. Exp Brain Res 2012;217:67-77.
- Côté JN, Patenaude I, St-Onge N, Fung J. Whiplash-associated disorders affect postural reactions to antero-posterior support surface translations during sitting. Gait Posture 2009;29:603-11.
- Peolsson A, Landén Ludvigsson M, Overmeer T, et al. Effects of neckspecific exercise with or without a behavioral approach in addition to prescribed physical activity for individuals with chronic whiplashassociated disorders: a prospective randomised study. BMC Musculoskelet Disord 2013;14:311.
- Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012;153:1144-7.
- Juul-Kristensen B, Clausen B, Ris I, et al. Increased neck muscle activity and impaired balance among females with whiplash-related chronic neck pain: a cross-sectional study. J Rehabil Med 2013;45:376-84.
- Peterson G, Nilsson D, Trygg J, et al. Novel insights into the interplay between ventral neck muscles in individuals with whiplash-associated disorders. Sci Rep 2015;5:1-13.
- Mayoux-Benhamou MA, Revel M, Vallée C, Roudier R, Barbet JP, Bargy F. Longus colli has a postural function on cervical curvature. Surg Radiol Anat 1994;16:367-71.
- Panjabi MM. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J Spinal Disord 1992;5:383-9; discussion 397.
- Peterson BW. Current approaches and future directions to understanding control of head movement. Prog Brain Res 2004;143:369-81.
- Elliott JM, O’Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine (Phila Pa 1976) 2010;35:948-54.
- Jull G, Amiri M, Bullock-Saxton J, Darnell R, Lander C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 1: Subjects with single headaches. Cephalagia 2007;27:793-802.
- Jull G, Falla D. Does increased superficial neck flexor activity in the craniocervical flexion test reflect reduced deep flexor activity in people with neck pain? Man Ther 2016;25:43-7.
- Zito G, Jull G, Story I. Clinical tests of musculoskeletal dysfunction in the diagnosis of cervicogenic headache. Man Ther 2006;11:118-29.
- Amiri M, Jull G, Bullock-Saxton J, Darnell R, Lander C. Cervical musculoskeletal impairment in frequent intermittent headache. Part 2: subjects with concurrent headache types. Cephalalgia 2007;28:891-8.
- Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Development of motor system dysfunction following whiplash injury. Pain 2003;103:65-73.
- Treleaven J. Dizziness, unsteadiness, visual disturbances, and postural control: implications for the transition to chronic symptoms after a whiplash trauma. Spine (Phila Pa 1976) 2011;36(25 Suppl):S211-7.
- Treleaven J, Chen X, Sarig Bahat H. Factors associated with cervical kinematic impairments in patients with neck pain. Man Ther 2016;22:109-15.
- Woodhouse A, Vasseljen O. Altered motor control patterns in whiplash and chronic neck pain. BMC Musculoskelet Disord 2008;9:90.
- Falla D, O’Leary S, Farina D, Jull G. Association between intensity of pain and impairment in onset and activation of the deep cervical flexors in patients with persistent neck pain. Clin J Pain 2011;27:309-14.
- Jull G, Sterling M, Kenardy J, Beller E. Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash?–A preliminary RCT. Pain 2007;129:28-34.
- Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain 2006;122:102-8.
- Jull GA, O’Leary SP, Falla DL. Clinical assessment of the deep cervical flexor muscles: the craniocervical flexion test. J Manipulative Physiol Ther 2008;31:525-33.
- Harris KD, Heer DM, Roy TC, Santos DM, Whitman JM, Wainner RS. Reliability of a measurement of neck flexor muscle endurance. Phys Ther 2005;85:1349-55.
- O’Leary S, Falla D, Jull G, Vicenzino B. Muscle specificity in tests of cervical flexor muscle performance. J Electromyogr Kinesiol 2007;17:35-40.
- Domenech MA, Sizer PS, Dedrick GS, McGalliard MK, Brismee JM. The deep neck flexor endurance test: normative data scores in healthy adults. PM R 2011;3:105-10.
- Jull G, Kristjansson E, Dall’Alba P. Impairment in the cervical flexors: a comparison of whiplash and insidious onset neck pain patients. Man Ther 2004;9:89-94.
- Nederhand MJ, Hermens HJ, IJzerman MJ, Turk DC, Zilvold G. Cervical muscle dysfunction in chronic whiplash-associated disorder grade 2: the relevance of the trauma. Spine (Phila Pa 1976) 2002;15:1056-61.
- Helgadottir H, Kristjansson E, Einarsson E, Karduna A, Jonsson H Jr. Altered activity of the serratus anterior during unilateral arm elevation in patients with cervical disorders. J Electromyogr Kinesiol 2011;21:9947-53.
- Tsang SM, Szeto GP, Lee RY. Altered spinal kinematics and muscle recruitment pattern of the cervical and thoracic spine in people with chronic neck pain during functional task. J Electromyogr Kinesiol 2014;24:104-13.
- Ebaugh DD, Spinelli BA. Scapulothoracic motion and muscle activity during the raising and lowering phases of an overhead reaching task. J Electromyogr Kinesiol 2010;20:199-205.
- Tsang SM, Szeto GP, Lee RY. Altered spinal kinematics and muscle recruitment pattern of the cervical and thoracic spine in people with chronic neck pain during functional task. J Electromyogr Kinesiol 2014;24:104-13.
- Kendall FP, McCreary EK, Provance PG, Rodgers M, Romani W. Muscles: Testing and Function, with Posture and Pain, 5th edn. Lippincott Williams & Wilkins, 2005.
- Rider LG, Koziol D, Giannini EH, et al. Validation of manual muscle testing and a subset of eight muscles (MMT8) for adult and juvenile idiopathic inflammatory myopathies. Arthritis Care Res (Hoboken) 2010;62:465-72.
- Bohannon RW. Manual muscle testing: does it meet the standards of an adequate screening test? Clin Rehabil 2005;19:662-67.
- Williams DA, Roush JR, Davies GJ, Ellenbecker TS, Rauh MJ. Alternative methods for measuring scapular muscles protraction and retraction maximal isometric forces. N Am J Sports Phys Ther 2009;4:200-9.
- Kumta P, MacDermid JC, Mehta SP, Stratford PW. The FIT-HaNSA demonstrates reliability and convergent validity of functional performance in patients with shoulder disorders. J Orthop Sports Phys Ther 2012;42:455-64.
- Pierrynowski M, McPhee C, P Mehta S, C MacDermid J, Gross A. Intra and inter-rater reliability and convergent validity of FIT-HaNSA in individuals with grade II whiplash associated disorder. Open Orthop J 2016;10:179-89.
- Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplashassociated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976) 2006;31:E847-55.
- Elliott J, Sterling M, Noteboom JT, Treleaven J, Galloway G, Jull G. The clinical presentation of chronic whiplash and the relationship to findings of MRI fatty infiltrates in the cervical extensor musculature: a preliminary investigation. Eur Spine J 2009;18:1371-8.
- O’Leary S, Cagnie B, Reeve A, Jull G, Elliott JM. Is there altered activity of the extensor muscles in chronic mechanical neck pain? A functional magnetic resonance imaging study. Arch Phys Med Rehabil 2011;92:929-34.
- Edmondston SJ, Wallumrød ME, Macléid F, Kvamme LS, Joebges S, Brabham GC. Reliability of isometric muscle endurance tests in subjects with postural neck pain. J Manipulative Physiol Ther 2008;31:348-54.
- Edmondston S, Björnsdóttir G, Pálsson T, Solgård H, Ussing K, Allison G. Endurance and fatigue characteristics of the neck flexor and extensor muscles during isometric tests in patients with postural neck pain. Man Ther 2011;16:332-8.
- Nijs J, Daenen L, Cras P, Struyf F, Roussel N, Oostendorp RA. Nociception affects motor output: a review on sensory-motor interaction with focus on clinical implications. Clin J Pain 2012;28:175-81.
- Sarig Bahat H, Sprecher E, Sela I, Treleaven J. Neck motion kinematics: an inter-tester reliability study using an interactive neck VR assessment in asymptomatic individuals. Eur Spine J 2016;25:2139-48.
- Shaw JW. Effect of a series of low-velocity whiplash-like perturbations on neck proprioception. In: Rehabiliation Science. Hamilton, McMaster University; 2008: p. 75.
- Walton DM, Macdermid JC, Nielson W, Teasell RW, Reese H, Levesque L. Pressure pain threshold testing demonstrates predictive ability in people with acute whiplash. J Orthop Sports Phys Ther 2011;41:658-65.
- Travell JG, Simons DG. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual, 2nd edn, Volumes 1 and 2. Baltimore, Maryland, USA: Williams and Wilkins, 1999.
- Pontinen PJ. Reliability, validity, reproducibility of algometry in diagnosis of active and latent tender spots and trigger points. J Musculoskel Pain 1998;6:61-71. 110
- Lucas N, Macaskill P, Irwig L, Moran R, Bogduk N. Reliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literature. Clin J Pain 2009;25:80-9.
- Walton DM, Levesque L, Payne M, Schick J. Clinical pressure pain threshold testing in neck pain: comparing protocols, responsiveness, and association with psychological variables. Phys Ther 2014;94:827-37.
- Hübscher M, Moloney N, Leaver A, Rebbeck T, McAuley JH, Refshauge KM. Relationship between quantitative sensory testing and pain or disability in people with spinal pain – A systematic review and metaanalysis. Pain 2013;154:1497-504.
