6 Pathogenesis of Chronic Whiplash-associated Disorder
6.1 Claudia’s Progression as a Person with Chronic Pain
Assessment of Claudia
Claudia, a friend of Amanda, experienced a whiplash injury four weeks ago as the seat-belted passenger in the car that Amanda was driving. Claudia worked as a clerk and smokes a couple of cigarettes per day. She had a one-year history of other musculoskeletal disorders – a shoulder tendinopathy and plantar fasciitis. Soon after the accident, Claudia was assessed in the emergency room, using the Canadian C-Spine rule (Figure 2-3). The Canadian C-spine rule is used in patients with acute trauma.1 After her assessment, Claudia was informed that her clinical problem was classified as WAD II. The grades of WAD are detailed in Chapter 1. Patients classified as WAD II do not suffer from any clinically identifiable source of nerve injury. However, newer experiments and investigations have demonstrated functional abnormalities involving the myofascial compartment of the head and neck. These may or may not have a structural injury component. We highlight such myofascial factors in Chapter 8.
Four weeks after the accident, Claudia continued to have high pain scores of 6 out of 10 on a numerical pain rating scale, indicating moderate to high intensity of pain, and high levels of disability on the Neck Disability Index (25 out of 50). She attended massage therapy, physiotherapy for gentle range of motion exercise, laser and manual therapy, acupuncture, and chiropractic treatment, all providing temporary relief. She could not tolerate more intensive activity-based physiotherapy due to pain with exercise. She believed that something was seriously wrong with her neck and that by avoiding exercise or activity she might prevent an increase in pain and further harm. She had requested additional X-rays from her family doctor. They were negative. She held and rubbed the right side of her neck frequently as she reported her story and symptoms. The area of her body with the most intense pain was on the right side of her neck and extended into her upper thoracic spine. She was exhausted and had interrupted sleep. She declined seeing a psychologist; she noted her pain is real. Her neck was sore to touch. She reported reduced range of motion with associated severe muscle spasm of her neck and shoulder girdle, and her pain appeared to have slowly spread to her head in the occipital region. She stated she needed further investigation, perhaps an MRI to identify what needs to be fixed. She hired a lawyer and is suing the truck driver. She was worried and anxious. She was increasingly unable to work, and became more sedentary. Her analgesic use increased and she had a low mood.
Claudia’s problem involved probable soft tissue injury, altered central processing, and central sensitization, and was characterized by bracing pain behaviors. Knowledge of altered central processing early in whiplash can help direct treatment for those with high pain, high disability, low self-efficacy and other prognostic indicators suggesting a poor or slow recovery. These indicators may suggest reduced chances of success with standard treatments and are shown to be associated with poor recovery.
6.2 The Risk of Chronicity in Whiplash Victims: The Physical Factors
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.2,3
6.2.1 Nociceptive Pain
Nociceptive pain refers specifically to the pain that is associated with the activation of the peripheral receptive terminals of primary afferent neurons in response to noxious chemical (inflammatory), mechanical, or thermal stimuli.4 An external stimulus leads to stimulation of specialized sensory end organs called nociceptors. These nociceptors are normally stimulated by various mechanical, chemical, and heat stimuli.5 The stimulation gives rise to signal transmission from periphery to central nervous system, after transduction to electrical signals at the periphery. A pain experience occurs in response to the incoming nociceptive signals.
6.2.2 Physiology of Pain Transmission
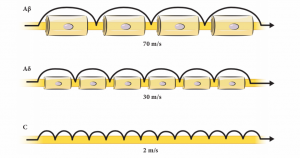
Normally two different fiber systems, the A-delta and C fibers, are involved in this process. They are pseudounipolar nerve fibers, with their central connections in the dorsal horn of the spinal cord, or nuclei of the brain stem (cranial nerves). The A-delta and C-fibers have some distinct properties, as highlighted in Figure 6-1 and Figure 6-2. A-delta fibers range in diameter from 1 to 6 μm and are covered by a thin layer of myelin. Apart from insulation and support, myelin enhances conduction velocity. They preferentially carry mechanical stimulation.6 The C-fiber axons are unmyelinated and preferentially respond to noxious heat. But they are also termed polymodal fibers. The A-beta fibers are normally involved in tactile stimulation, and are also involved in modulating nociceptive signals as they enter the dorsal horn. The A-beta fibers can also be involved in the process of sensitization, as explained below.7-9
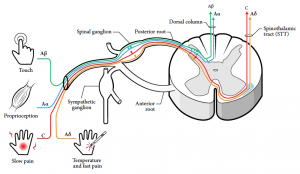
6.2.3 Sensitization of Pain
Peripheral noxious inputs, if sufficiently intense and prolonged, can give rise to altered and pathological hypersensitivity of nociceptors and non-nociceptor neurons. This mechanism has been termed as sensitization and occurs at both peripheral and central levels, for both acute and chronic pain conditions.10 The nociceptor-induced sensitization is usually reversible, adaptive, and serves a protective function.11 In the presence of ongoing nociceptive input the threshold for activation falls, and responses to subsequent stimuli are amplified, further enhancing the protective function. This is brought about by exposure of high-threshold primary sensory neurons to inflammatory mediators and damaged tissue.12 This enhancement of peripheral nociceptor traffic is termed peripheral sensitization, giving rise to primary hyperalgesia-hypersensitivity in and around the tissue injury.
In most conditions of chronic pain the peripheral noxious input is not present. However, there is still an enhancement in the functional status of neurons and synaptic connections throughout the neuraxis, in relation to the nociceptive pathways.11 Etiologically, the development of central sensitization leads to an indirect state of pain generation due to changes in the somatosensory system itself, rather than any ongoing noxious input. Clinically, these changes may manifest as allodynia – pain in response to a non-noxious stimuli (i.e., light touch); hyperalgesia – pain in response to a lower intensity stimuli that would usually cause pain only when the stimulus is applied at a higher intensity (e.g., increased sensitivity to pressure); and secondary hyperalgesia – a receptive field expansion that allows input from non-injured tissue to produce pain.13 These changes observed in central sensitization would involve myelinated A-beta fibers that are not normally involved with pain transmission. The presence and contribution of central sensitization to chronic pain conditions such as whiplash can be assessed by tests looking for mechanical and thermal sensitivity, allodynia, temporal summation, and referred pain.
6.2.4. Mechanism-based Pain Categorization
Categorization of pain based on the clinical dominance of the neurophysiological mechanisms underlying clinical presentations of pain features help to better understand a patient’s pain and also help direct suitable treatment strategies. The predominant mechanism can involve “nociceptive pain”, “neuropathic pain”, and “central sensitization”. Recently, attempts have been made to help clinicians translate this knowledge with either clinical guidelines for low back pain14 or musculoskeletal pain.4 Specific details on clinical indicators, criteria, and guidelines are provided in respective reference articles. Quantitative sensory testing (QST) can be used as an objective method to quantify and categorize the patient, based on underlying mechanisms.
6.2.5 Neuropathic Pain
According to the International Association for the Study of Pain (IASP) 1994 definition, neuropathic pain is defined as “pain initiated or caused by a primary lesion or dysfunction in the nervous system”.3 However, due to its broad nature, this definition lacked diagnostic specificity and anatomic precision.2,15 The definition was redefined in 2011 as, “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.” Two crucial but important differences include eliminating the word “dysfunction”, and substituting “somatosensory system” in the place of “nervous system”. This facilitates both clinicians and researchers to distinguish central sensitization (neuroplasticity), which happens with both neuropathic and non-neuropathic etiology, from actual neuropathic pain. Neuropathic pain is etiologically heterogeneous. Because there are no definitive diagnostic findings for neuropathic pain, a grading system with different levels of certainty has been proposed (see DID YOU KNOW? for criteria). There are four criteria to be assessed, with three levels of certainty – definite, probable, and possible.2 Considered from this perspective, chronic WAD I and II, which have been argued to have neuropathic components,16 would seem to have predominant central sensitization rather than neuropathic pain.
Screening questionnaires such as DN4,17 PainDETECT,18 or S-LANSS19,20 can be introduced during subjective interviewing to detect the presence of neuropathic characteristics (i.e., certain “qualities” of pain are associated with neuropathic pain to a greater extent than pain of other origins: pins/needles, tingling, numbness).
6.2.6. Whiplash and Central Sensitization
There is evidence that the mechanisms in whiplash involve central sensitization. As described above, this process involves persisting pain complaints in the absence of evident tissue damage, a common picture in patients with associated whiplash disorders.21 Central sensitization may involve temporal summation of pain or wind-up, increased activity of pain facilitatory pathways, malfunctioning of descending pain inhibitory mechanisms, altered sensory processing in the brain, and long-term potentiation of neuronal synapses in the anterior cingulate cortex.22
Did You Know?
Revised Neuropathic Pain Diagnostic Criteria and Grading System (adapted from Neurology 2008;70[18]:1630-5).2
The following criteria to be evaluated for each patient with suspected neuropathic pain:
- Pain with a distinct neuroanatomically plausible distribution (a region corresponding to a peripheral innervation territory or to topographic representation of a body part in the central nervous system).
- A history suggestive of a relevant lesion or disease affecting the peripheral or central somatosensory system (the suspected lesion or disease is reported to be associated with pain, including a temporal relationship typical for the condition).
- Demonstration of the distinct neuroanatomically plausible distribution by at least one confirmatory test (as part of the neurologic examination, these tests confirm the presence of negative or positive neurologic signs concordant with the distribution of pain). Clinical sensory examination may be supplemented by laboratory and objective tests to uncover subclinical abnormalities.
- Demonstration of the relevant lesion or disease by at least one confirmatory test (as part of the neurologic examination, these tests confirm the diagnosis of the suspected lesion or disease). These confirmatory tests depend on which lesion or disease is causing neuropathic pain.
Grading of certainty for the presence of neuropathic pain:
Definite neuropathic pain: all (1 to 4)
Probable neuropathic pain: 1 and 2, plus either 3 or 4
Possible neuropathic pain: 1 and 2, without confirmatory evidence from 3 or 4
REVIEW 6.1
Which of the following is false?
- C fibers are unmyelinated
- Proprioception is carried by C fibers
- Whiplash always involves neuropathic pain mechanisms
- Allodynia can be a feature of central sensitization
Correct answer: 2
6.3 Quantitative Sensory Testing
These are psychophysical tests using controlled stimuli that aid in identifying both hypo- and hyper-sensory functions, such as peripheral sensitization or central processing problems in central sensitization. QST as defined by the Peripheral Neuropathy Association are techniques used to measure the intensity of stimuli needed to produce specific sensory perceptions.23 Conventional nerve studies only assess large fiber functions, whereas QST can assess sensory processing of both large fibers (A-alpha and A-beta), and small afferent nerve fibers (A-delta and C-fibers). The large myelinated fibers are assessed with vibration and light touch, and small myelinated and unmyelinated fibers (A-delta and C-fibers) with pressure or thermal testing.24 By selecting different sensory inputs using QST assessment techniques, it is possible to evaluate the sensory processing of both large and small afferent nerve fibers.25,26 Allodynia is a pain response from stimuli that do not normally provoke pain, and can be classified into different types.
- Mechanical allodynia (A-delta and C-fiber mediated) including static mechanical allodynia – pain in response to light touch/pressure or dynamic mechanical allodynia – pain in response to light stroking.
- Thermal (hot or cold) allodynia (C and A-delta fiber mediated) – pain in response to minor changes in skin temperature within the affected area.
- Movement allodynia – pain triggered by normal movement of joints or muscles.
QST provides additional information on these psychophysical pain phenomena beyond self-report scales of pain intensity. It provides evidence of local or global hypersensitivity, facilitates a mechanistic evaluation and it can be used to measure change over time. QST can be performed reliably, using validated techniques. It is clinically feasible, and is an economical choice for clinicians.
Quantitative Sensory Testing
Clinically applicable examples of QST are presented below.
Pain pressure threshold
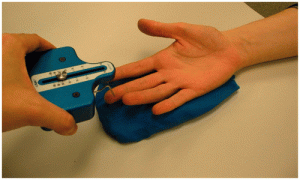
Pain Pressure Threshold (PPT) evaluates static mechanical allodynia (C and A-delta fibers mediated) using algometry27 and may identify hypersensitivity in distal points such as tibialis anterior likely indicating central sensitivity.28 Alteration of pain processing mechanisms (peripheral or central) may result in reduced PPT. Algometry is a means of assessing for primary (top image: upper fibers trapezius) or secondary (bottom image: tibialis anterior) hyperalgesia (Figure 6-3). In both acute and chronic problems, distal hypersensitivity likely indicates central sensitivity; a change score of at least 150 kPa on PPT is highly indicative of meaningful change (10 kPa = 1 N/cm2, so 150 kPa = 15 N change.28

Reliability:28 Inter-rater and test-retest, Excellent ICC=0.75–0.95. However, three measurements should be taken to maximize measurement properties of PPT.
Validity:28,29 Sensitivity (range 0.08–0.50); specificity (range 0.82–0.97). Depression, catastrophizing, and kinesiophobia (fear of movement) explain the small but statistically significant variance in upper fiber of trapezius (3.9%–5.9%).
Catastrophizing and kinesiophobia explain significant variance in the distal tibialis anterior PPT (3.6% and 2.9%).28 Spatial and temporal summation of pain evoked by mechanical pressure stimuli is clinically relevant in widespread pain.29
Predictive validity:30,31 PPT has prognostic utility in widespread hyperalgesia.
Responsiveness:28,30 SEM at upper fibers trapezius was 20.5 kPa30 for acute neck pain and 66.9 kPa for mixed neck pain.28 SEM at tibialis anterior was 42.3 kPa30 for acute neck pain and 43.9 kPa28 for mixed neck pain. Minimal detectable change at 90% confidence upper fibers trapezius was 157.60, and minimal detectable change at 90% confidence at tibialis anterior was 100.32 kPa.
Brushing
Brushing can be used to identify dynamic mechanical allodynia23,32 (pain with stroking of the skin; “feels like a sun burn”; C and A-delta fibers mediated) (Figure 6-4). This is often found in the region of most pain. Brushing assessed in the region of most pain may produce mechanical allodynia with stroking the skin and indicates central sensitization.

Content validity: QST33 was validated against evoked potentials or skin biopsy with generally good correlations for small fiber function. Skin biopsy was used to measure the intraepidermal nerve fiber density.
Immersion in Cold Water Evaluation
Immersion in Cold Water Evaluation (ICE) neural target is small (A-delta and C) fibers assessing thermal allodynia.34,35 ICE is administered through a standardized protocol where the hand is immersed in cold water and the examiner monitors the pain response on a Numeric Rating Scale just prior to and after immersion and monitors skin surface temperature rewarming similarly (Figure 6-5). A video demonstrating the ICE evaluation test can be found here.

Reliability:34 Test-retest–Excellent. ICC = 0.81 to 0.86 in healthy subjects; ICC = 0.79 to 0.82 in patients.
Construct validity:36 Moderate correlation between subjective reporting of cold intolerance using Numeric Rating Scale.
Discriminative validity:31,36,37 Cold responses are changed for WAD. Cold intolerance indicator of pain hypersensitivity.
Predictive validity:37,38 A prognostic factor for whiplash.
TEN Test
Neural targets of the TEN test are adaptive large (A-beta) fibers, and also small (A-delta) fibers.34,39,40 The normal and abnormal areas are simultaneously stroked with equal pressure maintained by examiner’s both fingers (Figure 6-6). The procedure produces an analog ratio of the abnormal body area compared with the normal area. A video demonstrating the TEN test can be found here.
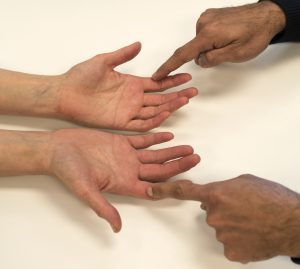
Reliability:41,42 Excellent inter-observer: ICC = 0.91;41 κ = 1.00, p<0.003;42 Good–excellent intra-observer: ICC = 0.62 to 0.90, p<0.05.41
Construct validity:36,43,44 Cold intolerance using Numeric Rating Scale is moderately correlated with the TEN test.
Concurrent validity:24,45 Comparison with other sensory testing techniques:45 Weinstein Enhanced Sensory Test (WEST) with calibrated monofilaments, static two-point discrimination, moving two-point discrimination, and Pressure-Specified Sensory Device (PSSD).24
Two-point Discrimination and Monofilament Tests
Two-point discrimination or monofilaments (A-beta fibers) engage fast and slow-adapting receptors involved in pressure/touch perception and measure touch threshold. These modalities can be assessed using the WEST measured on an ordinal scale, the PSSD measured on a continuous scale, and a two-point discrimination device measured on an ordinal scale.46 The WEST device, a modernized version of the von Frey hair filaments, has five different diametric monofilaments (0.07 grams; 0.2 grams; 2 grams; 4 grams; 200 grams) (Figure 6-7). The monofilament classified as normal sensitivity is 2.83 grams. There are monofilament sets with greater number of monofilament for more specific testing capacity. The monofilament is applied five times on different parts of the hand typically in radial, ulnar, and median nerve distributions (Figure 6-7). The PSSD has two prongs, each having a hemispherical tip with an area of 0.9 mm2 attached to a computerized force transducer (Figure 6-8). Using the PSSD, two-point discrimination is measured using a single or double prong on the hand. A Disk-Criminator tool can also be used to test static or dynamic two-point discrimination (Figure 6-9); can the client discriminate the difference between two points and one point? The test starts at 8 mm and goes down to 2 mm. Established reliability, validity, and responsiveness to change in systematic reviews are as follows.46,47 Test selection may depend on test availability and purpose.46
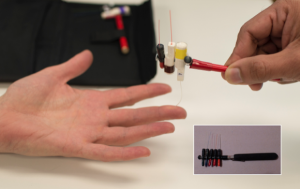
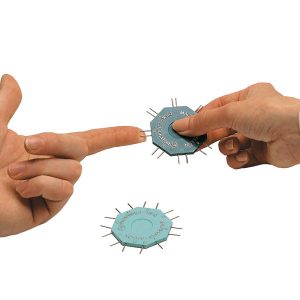
WEST
Concurrent validity: Von Frey/Semmes–Weinstein, Monofilaments correlate with: patient’s estimated impact on activities of daily living (r=0.59); object recognition time (r=0.70); object identification (r=0.55).
Responsiveness: Patients at intermediate term and long-term post-repair effect size=1.2 (large) at highest relative responsiveness when contrasted to localization, two-point discrimination, and pick up and object recognition.
PSSD
Concurrent validity: PSSD test has high sensitivity, but a low specificity in comparison with electrodiagnostic testing47 and has advantages over electrodiagnostic testing in brachial plexus injury.48 While it has a significant correlation with the small-object subset of the Mayo Dexterity Test (p<0.006) and with the object recognition test (p<0.001),49 it has low-moderate correlations (rs=0.32) with NK Dexterity Small Objects Test scores.50
Responsiveness: While PSSD has a clinically important difference of 0.15 g/mm2, it is not as discriminative and has lower responsiveness compared to a Vibrometer for patients with a clinically important improvement in symptoms.50
Two-point Discrimination
Concurrent validity:47 Static two-point discrimination has strong correlation with number of objects recognized (r=0.78); average recognition time (r=0.36); object recognition (r=0.77); and object identification (r=0.74). Moving two-point discrimination has strong correlation with number of objects recognized (r=0.87); object recognition (r=0.77); and object identification (r=0.66). Pick-up test (strong correlation); recognition of shapes (strong correlation).
Predictive validity:47 Moving two-point discrimination has very weak correlation with combined functional score (r=0.01) when controlling for age, delay, and follow-up. Static two-point discrimination has weak correlation with pick-up test (r=0.22) and object recognition (r=0.28) when controlling for age, delay, and follow-up.
Responsiveness:47 Patients with median and ulnar nerve injury, effect size=0.0 (no responsiveness). Patients at 6 to 18 months post repair, effect size=0.1 (very small responsiveness). Patients with ulnar and median nerve injury assessed over three occasions (low responsiveness), relative low responsiveness when contrasted against localization, touch threshold, and pick up and object recognition.
6.4 The Risk of Chronicity in a Whiplash Victim: The Non-physical Factors
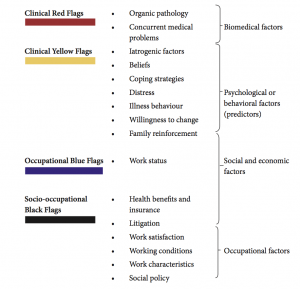
Claudia’s history was suggestive of chronic symptoms of pain, dysfunction, and an inability to cope. According to the present evidence, the risk of chronicity and suffering in a patient with axial pain is significantly affected by the presence of risk factors in various settings of a patient’s life. The assessment and triaging of these risk factors begin from the time of initial assessment and continues in each stage. These risk factors could be considered as colored flags (Figure 6-10), each indicating a particular domain or perspective of daily life; such as clinical (red)—as considered above, psychological (yellow), occupational (blue), and socio-occupational (black).51
6.4.1 Identification of Psychological Factors
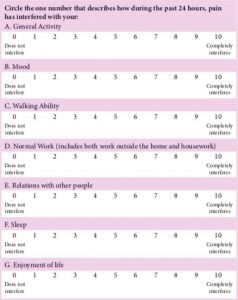
Yellow flags are psychosocial or behavioral factors indicating or predicting an increase in work loss, and the risk of long-term disability. The term yellow flag was coined by Kendall et al.52,53 Identification of such factors depends upon the extent to which a physician intends to diagnose and modify them. Simple, but specific questions can be utilized in the initial patient interview. The use of a simple self-administered questionnaire (not requiring physical measurement) that concentrates on physical symptoms and their effect on work and leisure activities and psychological well-being is the most useful tool in the evaluation of these patients. Such self assessment tools of interference with daily activities include the Brief Pain Inventory54 (BPI link) (Figure 6-11), and Pain Disability Index (PDI link).55 The reciprocal nature of the relationship between pain and psychological factors reviewed has shown that there is a clear link between these two factors. According to Linton, there is level A evidence supporting the association of psychological factors and reported onset of back and neck pain.56 According to the bio-psycho-social model of chronic pain, patients with the presence of these factors indicate the highest chances of poor recovery. The following psychological factors are influential:
- Pain catastrophizing57
- Fear of movement58
- Lower self-efficacy59
- Distress60
- Coping strategies61 – conflicting evidence
- Post-traumatic stress symptoms62-64
These factors can be linked to management (Table 6-1). Further details on psychological management of whiplash patients, including identification of risk factors are highlighted in Chapter 9.
6.4.2 Identification of Black and Blue Flags
Claudia reported that she must return to full duties; she was at risk of losing her job if this was not attainable. She was distressed and fearful about losing her job. These are black or blue flags. The modified integrative bio-psycho-social model of Waddell & Burton–2004,65 and the International Classification Function, Disability and Health (ICF) model66 are combined to help clinicians depict the physical suffering and functional impairment symptoms, presented and common to WAD. The ICF is a practical tool to elicit and track information on the functioning, disability, and participation of an individual within their contextual and environmental framework. Figure 6-12 depicts Claudia’s ICF profile.
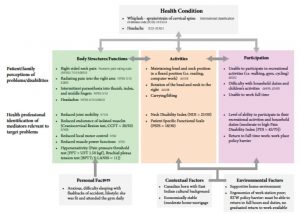
TABLE 6.1 Theory, mechanism, and intervention for chronic pain patients
| Theory | Mechanism | Practitioner Intervention (PT, MD, etc.) |
| Fear avoidance | Activity avoidance, disability, isolation | Graded activity; pain education68-70 |
| Acceptance and commitment | Failed attempts to control pain, frustration | Realistic goal setting;71-74 managed expectations; pain education |
| Misdirected problem-solving | Hypervigilance and rumination | Redirection toward functional goals; pain education |
| Self-efficacy | Reduced perceived self-control of pain | Encourage self-management; reduce dependence |
| Stress-diathesis | Distress predisposes or exacerbates pain | Stress-evaluation techniques, e.g., breathing |
MD, medical doctor; PT, physiotherapist
6.5 Management Considerations for Chronic Pain Patients with Whiplash
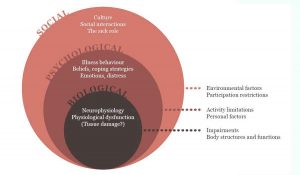
Patients with a more complex clinical presentation (moderate to severe pain and disability; movement loss; motor deficit; central sensitization and hyperexcitability; post-traumatic stress symptoms; higher level of psychological distress) will require more concerted management that includes psychological referral, pain relief, and encouragement of movements, taking care to avoid symptom provocation. The complexity of psychology and pain experience is depicted in Figure 6-13. Thus, linking theory, mechanism and treatment is important to the entire treatment team – the physician, psychologist, and physiotherapist (Table 6-1).67
6.6 References
- Stiell IG, Wells GA, Vandemheen KL, et al. The Canadian C-spine Rule for radiography in alert and stable trauma patients. JAMA 2001;286:1841-8.
- Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008;70(18):1630-5.
- Merskey H, Bogduk N, eds. Classification of Chronic Pain. 2nd edition. IASP Press: IASP, 1994.
- Smart KM, Blake C, Staines A, Doody C. Clinical indicators of ‘nociceptive’, ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man Ther 2010;15:80-7.
- Ekman EF, Koman LA. Acute pain following musculoskeletal injuries and orthopaedic surgery: mechanisms and management. Instr Course Lect 2005;54:21-33.
- Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis 1998;5:209-27.
- Loeser JD, Butler SH, Chapman CR, Turk DC, eds. Bonica’s Management of Pain. 3rd edition. Philadelphia: Lippincott Williams & Wilkins, 2001.
- Bourne S, Machado AG, Nagel SJ. Basic anatomy and physiology of pain pathways. Neurosurg Clin North Am 2014;25:629-38.
- Kaye AD, Urman RD, Vadivelu N, eds. Essentials of Regional Anesthesia. Springer Science+Business Media, LLC: Springer-Verlag New York, 2012.
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983;306:686-8.
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895-926.
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron 2007;55:365-76.
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2-15.
- Nijs J, Apeldoorn A, Hallegraeff H, et al. Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician 2015;18:E333-46.
- Jensen TS, Baron R, Haanpaa M, et al. A new definition of neuropathic pain. Pain 2011;152:2204-5.
- Davis CG. Mechanisms of chronic pain from whiplash injury. J Forensic Leg Med 2013;20:74-85.
- Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29-36.
- Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20.
- Bennett MI SB, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain 2005;6:9.
- Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147-57.
- Herren-Gerber R, Weiss S, Arendt-Nielsen L, et al. Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med (Malden, Mass) 2004;5:366-76.
- Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain 2013;17:299-312.
- Quantitative sensory testing: a consensus report from the Peripheral Neuropathy Association. Neurology 1993;43:1050-2.
- Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am 2003;14:261-86.
- Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 2006;10:77-88.
- Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123:231-43.
- Tunks E, Crook J, Norman G, Kalaher S. Tender points in fibromyalgia. Pain 1988;34:11-9.
- Walton DM, Levesque L, Payne M, Schick J. Clinical pressure pain threshold testing in neck pain: comparing protocols, responsiveness, and association with psychological variables. Phys Ther 2014;94:827-37.
- Nie HL, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal summation of pain evoked by mechanical pressure stimulation. Eur J Pain 2009;13:592-99.
- Walton DM, MacDermid JC, Nielson W, et al. Pressure pain threshold testing demonstrates predictive ability in people with acute whiplash. J Orthop Sports Phys Ther 2011;41:658-65.
- Sterling M, Jull G, Vicenzino B, Kenardy J, Darnell R. Physical and psychological factors predict outcome following whiplash injury. Pain 2005;114:141-8.
- Marmura MJ, Abbas M, Ashkenazi A. Dynamic mechanical (brush) allodynia in cluster headache: a prevalence study in a tertiary headache clinic. J Headache Pain 2009;10:255-8.
- Haanpää M, Attal N, Backonja M. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011;152:14-27.
- Uddin Z, MacDermid J, Packham T. Clinical implementation of two quantitative Sensory Tests: Cold Stress Test and the Ten Test. Physiother Pract Res 2014;35:33-40.
- Courtney CA, Kavchak AE, Lowry CD, O’Hearn MA. Interpreting joint pain: quantitative sensory testing in musculoskeletal management. J Orthop Sports Phys Ther 2010;40:818-25.
- Maxwell S, Sterling M. An investigation of the use of a numeric pain rating scale with ice application to the neck to determine cold hyperalgesia. Man Ther 2013;18:172-74.
- Goldsmith R, Wright C, Bell SF, Rushton A. Cold hyperalgesia as a prognostic factor in whiplash associated disorders: A systematic review. Man Ther 2012;17:402-10.
- Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122:102-8.
- Uddin Z, MacDermid J, Packham T. The Ten test for sensation. J Physiother 2013;59:132.
- Uddin Z, Macdermid J, Packham T. Ice-water (cold stress) immersion testing. J Physiother 2013;59:277.
- Strauch B, Lang A, Ferder M, Keyes-Ford M, Freeman K, Newstein D. The Ten Test. Plast Reconstr Surg 1997;99:1074.
- Sun HH, Oswald TM, Sachanandani NS, Borschel GH. The Ten test: application and limitations in assessing sensory function in the paediatric hand. J Plast Reconstr Aesthet Surg 2010;63:1849-52.
- Traynor R, MacDermid J. Immersion in cold-water evaluation (ICE) and self-reported cold intolerance are reliable but unrelated measures. Hand 2008;3:212-19.
- MacDermid J, Gross AR, Galea V, McLaughlin LM, Parkinson WL, Woodhouse L. Developing biologically-based assessment tools for physical therapy management of neck pain. J Sports Physical Ther 2009;39:388-99.
- Patel MR, Bassini LA. A comparison of five tests for determining hand sensibility. J Reconstr Microsurg 1999;15:523-6.
- Uddin Z, MacDermid JC, Ham HH. Test–retest reliability and validity of normative cut-offs of the two devices measuring touch threshold: Weinstein Enhanced Sensory Test and Pressure-Specified Sensory Device. Hand Ther 2014;19:3-10.
- Tassler P, Dellon A. Correlation of measurements of pressure perception using the pressure-specified sensory device with electrodiagnostic testing. J Occup Environ Med 1995;37:862-6.
- Nath RK, Bowen ME and Eichhorn MG. Pressure-specified sensory device versus electrodiagnostic testing in brachial plexus upper trunk injury. J Reconstr Microsurg 2010;26:235-42.
- Dellon ES, Keller KM, Moratz V, Dellon AL. Validation of cutaneous pressure threshold measurements for the evaluation of hand function. Ann Plastic Surg. 1997;38:485-92
- Cheung DK, MacDermid JC, Walton D, Grewal R. The construct validity and responsiveness of sensory tests in patients with carpal tunnel syndrome. Open Orthop J 2014;8:100-7.
- Main CJ, Williams AC. Musculoskeletal pain. BMJ (Clinical research ed) 2002;325:534-7.
- Nicholas MK, Linton SJ, Watson PJ, Main CJ. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther 2011;91:737-53.
- Kendall NA, Linton SJ, Main CJ. Guide to Assessing Psychosocial Yellow Flags in Acute Low Back Pain: Risk Factors for Long-Term Disability and Work Loss. Wellington, New Zealand: Accident Rehabilitation and Compensation Insurance Corporation of New Zealand and the National Health Committee; 1997.
- Bortsov AV, Platts-Mills TF, Peak DA, et al. Effect of pain location and duration on life function in the year after motor vehicle collision. Pain 2014;155:1836-45.
- Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil 1987;68:438-41.
- Linton SJ. A review of psychological risk factors in back and neck pain. Spine 2000;25:1148-56.
- Sullivan MJ, Stanish W, Sullivan ME, Tripp D. Differential predictors of pain and disability in patients with whiplash injuries. Pain Res Manag 2002;7:68-74.
- Vangronsveld KL, Peters M, Goossens M, Vlaeyen J. The influence of fear of movement and pain catastrophizing on daily pain and disability in individuals with acute whiplash injury: a daily diary study. Pain 2008;139:449-57.
- Bunketorp-Kall LS, Andersson C, Asker B. The impact of subacute whiplash-associated disorders on functional self-efficacy: a cohort study. Int J Rehabil Res 2007;30:221-6.
- Carstensen TBW, Frostholm L, Oernboel E, et al. Post-trauma ratings of pre-collision pain and psychological distress predict poor outcome following acute whiplash trauma: A 12-month follow-up study. Pain 2008;139:248-59.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87.
- Sterling M, Hendrikz J, Kenardy J. Similar factors predict disability and posttraumatic stress disorder trajectories after whiplash injury. Pain 2011;152:1272-8.
- Kenardy J, DunneR. Traumatic injury and traumatic stress. Spine 2011;36(25 Suppl):S233-7.
- Rosenfeld LC, Nguyen D, Lenehan T, et al. Exercise augmentation compared to usual care for post traumatic stress disorder: A randomized controlled trial. BMC Psychiatry 2011;11:115.
- Waddell G, Burton AK. Concepts of Rehabilitation for the Management of Common Health Problems. London: The Stationery Office, 2004.
- World Health Organization. International Classification of Functioning, Disability and Health. Geneva: World Health Organization.
- Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Phys Ther 2011;91:700-11.
- Louw A, Diener I, Butler DS, Puentedura EJ. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain Archives Physical Med Rehabil 2011;92:2041-56.
- van Oosterwijck J, Nijs J, Meeus M, et al. Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study. J Rehabil Res Dev 2011;48:43-58.
- Meeus M, Nijs J, van Oosterwijck J, van Alsenoy V, Truijen S. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education: a doubleblind randomized controlled trial. Arch Physical Med Rehabil 2010;91:1153-9.
- Busch AJ, Schachter CL, Overend TJ, Peloso PM, Barber KR. Exercise for fibromyalgia: A systematic review. J Rheumatol 2008;35:1130-44.
- van Middelkoop M, Rubinstein SM, Kuijpers T, et al. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J 2011;20:19-39.
- Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil 2015;29:1155-67.
- Gross A, Kay TM, Paquin JP, et al. Exercises for mechanical neck disorders. Cochrane Database Syst Rev 2015;1:CD004250.
