Part 1 – Refrigerants
Thermodynamic efficiency of a refrigeration system depends mainly on its operating temperatures. Other important factors include: the system design, size, initial and operating costs, safety, reliability, and serviceability.
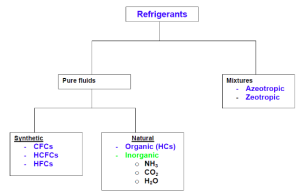
Primary and Secondary Refrigerants:
Refrigerants can be classified into primary and secondary refrigerants.
| Primary Refrigerants
Those fluids, which are used directly as working fluids
|
Secondary Refrigerants
Those liquids used for transporting thermal energy from one location to other.
|
NOTE: If the operating temperatures are above 0oC, then pure water can also be used as secondary refrigerant.
Ideal Refrigerant
So what properties make a refrigerant ideal? A refrigerant should possess the following 11 properties:
- Low boiling temperature at atmospheric pressure
- High latent heat capacity
- Moderate condensing temperature at a relatively low pressure
- Low specific volume, at compressor suction pressure, to minimize the required compressor size
- Ideal Refrigerant
- Stable chemical composition
- Inoffensive odour that will not taint stored goods
- Non-poisonous nature
- Non-corrosive properties
- Non-flammable and non-explosive nature when mixed with air
- Low cost and readily available
Let’s take a closer look at some of the good thermodynamic properties that make an ideal refrigerant.
Activity: Ideal Refrigerant
Click the accordion tabs to learn more about good thermodynamic properties of the ideal refrigerant.
Now that we’ve looked at good thermodynamic properties, let’s take a look at Environmental and safety properties for an ideal refrigerant.
Ozone Depletion Potential (ODP):
According to the Montreal protocol, the ODP of refrigerants should be zero, i.e., they should be non-ozone depleting substances.
Global Warming Potential (GWP):
Should have as low a GWP value as possible to minimize the problem of global warming.
Ozone Depletion Potential (ODP):
A number that refers to the amount of ozone depletion caused by a substance. Ratio of the impact on ozone of a chemical compared to the impact of a similar mass of CFC-11.
- The ODP of CFC-11 is defined to be 1.0.
- Other CFCs and HCFCs have ODPs that range from 0.01 to 1.0.
- HFCs have zero ODP because they do not contain chlorine.
Global Warming Potential (GWP):
- A number that refers to the amount of global warming caused by a substance
- Ratio of the warming caused by a substance to the warming caused by a similar mass of carbon dioxide.
- GWP of CO2 is 1.0
- CFC-12 has a GWP of 8,500
- CFC-11 has a GWP of 5,000
Refrigeration Systems and Equipment:
| Refrigerant | Ozone Depletion Potential (ODP) |
Global Warming Potential (GWP) |
| R-11 Trichlorofluoromethane | 1.0 | 4000 |
| R-12 Dichlorodifluoromethane | 1.0 | 2400 |
| R-22 Chlorodifluoromethane | 0.05 | 1700 |
| R-134a Tetrafluoroethane | 0 | 1300 |
| R-143a Trifluoroethane | 0 | 4300 |
| R-245a Pentafluoropropane | 0 | |
| R-717 Ammonia – NH3 | 0 | 0 |
| R-718 Water – H20 | 0 | |
| R-729 Air | 0 | |
| R-744 Carbon Dioxide – CO2 | 1 |
Total Equivalent Warming Index (TEWI): The factor TEWI considers both direct (due to release into atmosphere) and indirect (through energy consumption) contributions of refrigerants to global warming
Toxicity: Refrigerants used in a refrigeration system should be non-toxic.
Flammability: Should preferably be non-flammable and non-explosive.
Chemical stability: Should be chemically stable as long as they are inside the refrigeration system.. Compatibility with common materials of construction
Miscibility with lubricating oils: Oil separators have to be used if the refrigerant is not miscible with lubricating oil (e.g. ammonia)
Ease of leak detection: It should be easy to detect the leaks.
Now, let’s take a look at Types of Refrigerants
Types of Refrigerants
Some of the common refrigerants, in use today, are:
Refrigerant R-12( Dichorofluoromethane – CCl2F2)
Refrigerant R-22 (Chlorodifluoromethane – CHClF2)
Refrigerant R-134a (Tetrafluoroethane – C2H2F4)
Ammonia
Carbon dioxide
Sulphur dioxide
Ethyl chloride
Chlorofluorocarbons
Refrigerant Chemical Spotlight: Chlorofluorocarbons
Chlorofluorocarbons are odourless, non-toxic, non-flammable, and chemically inert. Now known to be partly responsible for the destruction of the ozone layer. Montréal Protocol was one of the first global environmental treaties and it banned the use of chemicals responsible for ozone damage, such as CFCs in aerosols and refrigerants.
CFCs react with ozone (O3) to form free chlorine (Cl) atoms and molecular oxygen (O2). Chlorine liberated during ozone breakdown can react with still more ozone. CFCs can remain in the atmosphere for more than a hundred years.
Designation of Refrigerants
1) Fully saturated, halogenated compounds: derivatives of alkanes (CnH2n+2) such as methane (CH4), ethane (C2H6)
-
- R-11 – CCl3F
- R-12 – CCl3F2
2)Inorganic refrigerants: Designated by number 7 followed by the molecular weight of the refrigerant (rounded-off).
-
- Ammonia:
- Molecular weight is 17, ∴ the designation is R 717
- Carbon dioxide:
- Molecular weight is 44, ∴ the designation is R 744
- Water:
- Molecular weight is 18, ∴ the designation is R 718
- Ammonia:
3) Mixtures: Mixtures of different refrigerants
-
-
- Azeotropic mixtures are designated by 500 series
- Zeotropic refrigerants are designated by 400 series.
- Azeotropic mixtures:
- R 500: Mixture of R 12 (73.8 %) and R 152a (26.2%)
- R 502: Mixture of R 22 (48.8 %) and R 115 (51.2%)
- R503: Mixture of R 23 (40.1 %) and R 13 (59.9%)
- R507A: Mixture of R 125 (50%) and R 143a (50%)
- Zeotropic mixtures:
- R404A : Mixture of R 125 (44%), R 143a (52%) and R 134a (4%)
- R407A : Mixture of R 32 (20%), R 125 (40%) and R 134a (40%)
- R407B : Mixture of R 32 (10%), R 125 (70%) and R 134a (20%)
- R410A : Mixture of R 32 (50%) and R 125 (50%)
-
4) Hydrocarbons:
-
- Propane (C3H8) : R 290
- n-butane (C4H10) : R 600
- iso-butane (C4H10) : R 600a
- Unsaturated Hydrocarbons: R1150 (C2H4)
- R1270 (C3H6)
Refrigerant Classes:
-
- 000 Methane-based – R12 CCl3F2
- 100 Ethane-based – R112 CCl2FCCl2F
- 200 Propane-based – R216 CF3CCl2CF3
- 300 Cyclic organic
- 400 Zeotropes
- 500 Azeotropes
- 600 Organic
- 700 Inorganic
- 1000 Unsaturated organic
Ozone Depletion Potential (ODP):
Restricted refrigerants commonly used for refrigeration, cold storage and air conditioning applications:
- R-11 (CFC 11), R-12 (CFC 12), R-22 (HCFC 22),
- R-502 (CFC 12 + HCFC 22)
Synthetic replacements for the older refrigerants are:
-
- R-134a (HFC-134a)
- Blends of HFCs
Synthetic refrigerants are non-toxic and non-flammable.
-
- Also have higher Global Warming Potential (GWP)
Natural Refrigerants:
Most commonly used natural refrigerant is ammonia. Ammonia is one of the oldest known refrigerants for it’s good thermodynamic, thermophysical and environmental properties. However, Ammonia is toxic and is not compatible with some of the common materials of construction such as copper.
Alternate Refrigerants
Classified into two broad groups:
-
-
- Non-ODS, synthetic refrigerants based on Hydro-Fluoro-Carbons (HFCs) and their blends
- Natural refrigerants including ammonia, carbon dioxide, hydrocarbons and their blends
-
| Refrigerants | ||
| Low Pressure | R11 | Trichlorofluoromethane |
| R13 | Chlorotrifluoromethane | |
| R113 | Trichlorotrifluoroethane | |
| R123 | Dichlorotrifluoroethane | |
| Medium Pressure | R114 | 1,2-dichloro-1,1,2,2-tetrafluoroethane |
| High Pressure | R12 | Dichlorodifluoromethane |
| R22 | Chlorodifluoromethane | |
| R134a | Tetrafluoroethane | |
| R410A | Difluoromethane/Pentafluoroethane | |
| R500 | Dichlorodifluoromethane/ Difluoroethane |
|
| R502 | Chlorodifluoromethane/ Chloropentafluoroethane |
Non-CO2 Refrigerants
Natural: Refrigerants such as the greenpeace-developed ‘Greenfreeze’. There are based on purified butane/propane mixtures. But are they entirely ‘natural‘? Due to increased efficiency over refrigerants such as R134a, allow the use of very small amounts of refrigerant to be used.
Reduced environmental damage: Hydrocarbon refrigerants are backward compatible with car air conditioning systems thus increasing their efficiency, and preventing further release of harmful R-134a and R12 to the atmosphere. Pure hydrocarbon refrigerants also have a short lifetime in the atmosphere, weeks to months. CO2, being more chemically stable, persists longer in the atmosphere.
Refrigerant Trends:
Increasing carbon- Increases the molecular weight and the boiling point.
Increasing nitrogen- Makes the compound more reactive. This can lead to toxicity and instability issues.
Increasing oxygen- Reduces atmospheric stability. Good for GWP and ODP but may lead to toxicity, flammability and reactivity issues.
Increasing sulphur- Increases toxicity and decreases stability.
Increasing hydrogen- Reduces atmospheric lifetime, which is good for GWP and ODP but increases flammability.
Increasing fluorine- Attached to carbon increases GWP.
Increasing chlorine- Improves lubricant miscibility but also ODP and toxicity.
Increasing bromine- Increases ODP but lowers flammability.
