12 Conditioning and Learning
Original chapter by Mark E. Bouton adapted by the Queen’s University Psychology Department
This Open Access chapter was originally written for the NOBA project. Information on the NOBA project can be found below.
We encourage students to use the “Three-Step Method” for support in their learning. Please find our version of the Three-Step Method, created in collaboration with Queen’s Student Academic Success Services, at the following link: https://sass.queensu.ca/psyc100/
Basic principles of learning are always operating and always influencing human behavior. This module discusses the two most fundamental forms of learning — classical (Pavlovian) and instrumental (operant) conditioning. Through them, we respectively learn to associate 1) stimuli in the environment, or 2) our own behaviors, with significant events, such as rewards and punishments. The two types of learning have been intensively studied because they have powerful effects on behavior, and because they provide methods that allow scientists to analyze learning processes rigorously. This module describes some of the most important things you need to know about classical and instrumental conditioning, and it illustrates some of the many ways they help us understand normal and disordered behavior in humans. The module concludes by introducing the concept of observational learning, which is a form of learning that is largely distinct from classical and operant conditioning.
Learning Objectives
- Distinguish between classical (Pavlovian) conditioning and instrumental (operant) conditioning.
- Understand some important facts about each that tell us how they work.
- Understand how they work separately and together to influence human behavior in the world outside the laboratory.
- Students will be able to list the four aspects of observational learning according to Social Learning Theory.
Two Types of Conditioning
Although Ivan Pavlov won a Nobel Prize for studying digestion, he is much more famous for something else: working with a dog, a bell, and a bowl of saliva. Many people are familiar with the classic study of “Pavlov’s dog,” but rarely do they understand the significance of its discovery. In fact, Pavlov’s work helps explain why some people get anxious just looking at a crowded bus, why the sound of a morning alarm is so hated, and even why we swear off certain foods we’ve only tried once. Classical (or Pavlovian) conditioning is one of the fundamental ways we learn about the world around us. But it is far more than just a theory of learning; it is also arguably a theory of identity. For, once you understand classical conditioning, you’ll recognize that your favorite music, clothes, even political candidate, might all be a result of the same process that makes a dog drool at the sound of bell.

Around the turn of the 20th century, scientists who were interested in understanding the behavior of animals and humans began to appreciate the importance of two very basic forms of learning. One, which was first studied by the Russian physiologist Ivan Pavlov, is known as classical, or Pavlovian conditioning. In his famous experiment, Pavlov rang a bell and then gave a dog some food. After repeating this pairing multiple times, the dog eventually treated the bell as a signal for food, and began salivating in anticipation of the treat. This kind of result has been reproduced in the lab using a wide range of signals (e.g., tones, light, tastes, settings) paired with many different events besides food (e.g., drugs, shocks, illness; see below).
We now believe that this same learning process is engaged, for example, when humans associate a drug they’ve taken with the environment in which they’ve taken it; when they associate a stimulus (e.g., a symbol for vacation, like a big beach towel) with an emotional event (like a burst of happiness); and when they associate the flavor of a food with getting food poisoning. Although classical conditioning may seem “old” or “too simple” a theory, it is still widely studied today for at least two reasons: First, it is a straightforward test of associative learning that can be used to study other, more complex behaviors. Second, because classical conditioning is always occurring in our lives, its effects on behavior have important implications for understanding normal and disordered behavior in humans.
In a general way, classical conditioning occurs whenever neutral stimuli are associated with psychologically significant events. With food poisoning, for example, although having fish for dinner may not normally be something to be concerned about (i.e., a “neutral stimuli”), if it causes you to get sick, you will now likely associate that neutral stimuli (the fish) with the psychologically significant event of getting sick. These paired events are often described using terms that can be applied to any situation.
The dog food in Pavlov’s experiment is called the unconditioned stimulus (US) because it elicits an unconditioned response (UR). That is, without any kind of “training” or “teaching,” the stimulus produces a natural or instinctual reaction. In Pavlov’s case, the food (US) automatically makes the dog drool (UR). Other examples of unconditioned stimuli include loud noises (US) that startle us (UR), or a hot shower (US) that produces pleasure (UR).On the other hand, a conditioned stimulus produces a conditioned response. A conditioned stimulus (CS) is a signal that has no importance to the organism until it is paired with something that does have importance. For example, in Pavlov’s experiment, the bell is the conditioned stimulus. Before the dog has learned to associate the bell (CS) with the presence of food (US), hearing the bell means nothing to the dog. However, after multiple pairings of the bell with the presentation of food, the dog starts to drool at the sound of the bell. This drooling in response to the bell is the conditioned response (CR). Although it can be confusing, the conditioned response is almost always the same as the unconditioned response. However, it is called the conditioned response because it is conditional on (or, depends on) being paired with the conditioned stimulus (e.g., the bell). To help make this clearer, consider becoming really hungry when you see the logo for a fast food restaurant. There’s a good chance you’ll start salivating. Although it is the actual eating of the food (US) that normally produces the salivation (UR), simply seeing the restaurant’s logo (CS) can trigger the same reaction (CR).
Another example you are probably very familiar with involves your alarm clock. If you’re like most people, waking up early usually makes you unhappy. In this case, waking up early (US) produces a natural sensation of grumpiness (UR). Rather than waking up early on your own, though, you likely have an alarm clock that plays a tone to wake you. Before setting your alarm to that particular tone, let’s imagine you had neutral feelings about it (i.e., the tone had no prior meaning for you). However, now that you use it to wake up every morning, you psychologically “pair” that tone (CS) with your feelings of grumpiness in the morning (UR). After enough pairings, this tone (CS) will automatically produce your natural response of grumpiness (CR). Thus, this linkage between the unconditioned stimulus (US; waking up early) and the conditioned stimulus (CS; the tone) is so strong that the unconditioned response (UR; being grumpy) will become a conditioned response (CR; e.g., hearing the tone at any point in the day—whether waking up or walking down the street—will make you grumpy). Modern studies of classical conditioning use a very wide range of CSs and USs and measure a wide range of conditioned responses.

Although classical conditioning is a powerful explanation for how we learn many different things, there is a second form of conditioning that also helps explain how we learn. First studied by Edward Thorndike, and later extended by B. F. Skinner, this second type of conditioning is known as instrumental or operant conditioning. Operant conditioning occurs when a behavior (as opposed to a stimulus) is associated with the occurrence of a significant event. In the best-known example, a rat in a laboratory learns to press a lever in a cage (called a “Skinner box”) to receive food. Because the rat has no “natural” association between pressing a lever and getting food, the rat has to learn this connection. At first, the rat may simply explore its cage, climbing on top of things, burrowing under things, in search of food. Eventually while poking around its cage, the rat accidentally presses the lever, and a food pellet drops in. This voluntary behavior is called an operant behavior, because it “operates” on the environment (i.e., it is an action that the animal itself makes).
Now, once the rat recognizes that it receives a piece of food every time it presses the lever, the behavior of lever-pressing becomes reinforced. That is, the food pellets serve as reinforcers because they strengthen the rat’s desire to engage with the environment in this particular manner. In a parallel example, imagine that you’re playing a street-racing video game. As you drive through one city course multiple times, you try a number of different streets to get to the finish line. On one of these trials, you discover a shortcut that dramatically improves your overall time. You have learned this new path through operant conditioning. That is, by engaging with your environment (operant responses), you performed a sequence of behaviors that that was positively reinforced (i.e., you found the shortest distance to the finish line). And now that you’ve learned how to drive this course, you will perform that same sequence of driving behaviors (just as the rat presses on the lever) to receive your reward of a faster finish.
Operant conditioning research studies how the effects of a behavior influence the probability that it will occur again. For example, the effects of the rat’s lever-pressing behavior (i.e., receiving a food pellet) influences the probability that it will keep pressing the lever. For, according to Thorndike’s law of effect, when a behavior has a positive (satisfying) effect or consequence, it is likely to be repeated in the future. However, when a behavior has a negative (painful/annoying) consequence, it is less likely to be repeated in the future. Effects that increase behaviors are referred to as reinforcers, and effects that decrease them are referred to as punishers.
An everyday example that helps to illustrate operant conditioning is striving for a good grade in class—which could be considered a reward for students (i.e., it produces a positive emotional response). In order to get that reward (similar to the rat learning to press the lever), the student needs to modify their behavior. For example, the student may learn that speaking up in class gets him/her participation points (a reinforcer), so the student speaks up repeatedly. However, the student also learns that s/he shouldn’t speak up about just anything; talking about topics unrelated to school actually costs points. Therefore, through the student’s freely chosen behaviors, s/he learns which behaviors are reinforced and which are punished.An important distinction of operant conditioning is that it provides a method for studying how consequences influence “voluntary” behavior. The rat’s decision to press the lever is voluntary, in the sense that the rat is free to make and repeat that response whenever it wants. Classical conditioning, on the other hand, is just the opposite—depending instead on “involuntary” behavior (e.g., the dog doesn’t choose to drool; it just does). So, whereas the rat must actively participate and perform some kind of behavior to attain its reward, the dog in Pavlov’s experiment is a passive participant. One of the lessons of operant conditioning research, then, is that voluntary behavior is strongly influenced by its consequences.
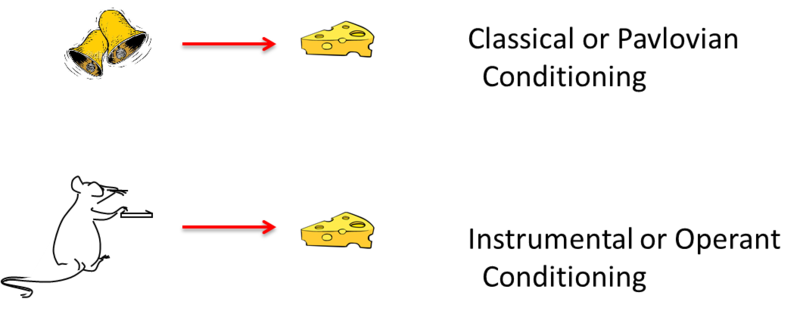
The illustration above summarizes the basic elements of classical and instrumental conditioning. The two types of learning differ in many ways. However, modern thinkers often emphasize the fact that they differ—as illustrated here—in what is learned. In classical conditioning, the animal behaves as if it has learned to associate a stimulus with a significant event. In operant conditioning, the animal behaves as if it has learned to associate a behaviorwith a significant event. Another difference is that the response in the classical situation (e.g., salivation) is elicited by a stimulus that comes before it, whereas the response in the operant case is not elicited by any particular stimulus. Instead, operant responses are said to be emitted. The word “emitted” further conveys the idea that operant behaviors are essentially voluntary in nature.
Understanding classical and operant conditioning provides psychologists with many tools for understanding learning and behavior in the world outside the lab. This is in part because the two types of learning occur continuously throughout our lives. It has been said that “much like the laws of gravity, the laws of learning are always in effect” (Spreat & Spreat, 1982).
Useful Things to Know about Classical Conditioning
Classical Conditioning Has Many Effects on Behavior
A classical CS (e.g., the bell) does not merely elicit a simple, unitary reflex. Pavlov emphasized salivation because that was the only response he measured. But his bell almost certainly elicited a whole system of responses that functioned to get the organism ready for the upcoming US (food) (see Timberlake, 2001). For example, in addition to salivation, CSs (such as the bell) that signal that food is near also elicit the secretion of gastric acid, pancreatic enzymes, and insulin (which gets blood glucose into cells). All of these responses prepare the body for digestion. Additionally, the CS elicits approach behavior and a state of excitement. And presenting a CS for food can also cause animals whose stomachs are full to eat more food if it is available. In fact, food CSs are so prevalent in modern society, humans are likewise inclined to eat or feel hungry in response to cues associated with food, such as the sound of a bag of potato chips opening, the sight of a well-known logo (e.g., Coca-Cola), or the feel of the couch in front of the television.
Classical conditioning is also involved in other aspects of eating. Flavors associated with certain nutrients (such as sugar or fat) can become preferred without arousing any awareness of the pairing. For example, protein is a US that your body automatically craves more of once you start to consume it (UR): since proteins are highly concentrated in meat, the flavor of meat becomes a CS (or cue, that proteins are on the way), which perpetuates the cycle of craving for yet more meat (this automatic bodily reaction now a CR).
In a similar way, flavors associated with stomach pain or illness become avoided and disliked. For example, a person who gets sick after drinking too much tequila may acquire a profound dislike of the taste and odor of tequila—a phenomenon called taste aversion conditioning. The fact that flavors are often associated with so many consequences of eating is important for animals (including rats and humans) that are frequently exposed to new foods. And it is clinically relevant. For example, drugs used in chemotherapy often make cancer patients sick. As a consequence, patients often acquire aversions to foods eaten just before treatment, or even aversions to such things as the waiting room of the chemotherapy clinic itself (see Bernstein, 1991; Scalera & Bavieri, 2009).
Classical conditioning occurs with a variety of significant events. If an experimenter sounds a tone just before applying a mild shock to a rat’s feet, the tone will elicit fear or anxiety after one or two pairings. Similar fear conditioning plays a role in creating many anxiety disorders in humans, such as phobias and panic disorders, where people associate cues (such as closed spaces, or a shopping mall) with panic or other emotional trauma (see Mineka & Zinbarg, 2006). Here, rather than a physical response (like drooling), the CS triggers an emotion.
Another interesting effect of classical conditioning can occur when we ingest drugs. That is, when a drug is taken, it can be associated with the cues that are present at the same time (e.g., rooms, odors, drug paraphernalia). In this regard, if someone associates a particular smell with the sensation induced by the drug, whenever that person smells the same odor afterward, it may cue responses (physical and/or emotional) related to taking the drug itself. But drug cues have an even more interesting property: They elicit responses that often “compensate” for the upcoming effect of the drug (see Siegel, 1989). For example, morphine itself suppresses pain; however, if someone is used to taking morphine, a cue that signals the “drug is coming soon” can actually make the person more sensitive to pain. Because the person knows a pain suppressant will soon be administered, the body becomes more sensitive, anticipating that “the drug will soon take care of it.” Remarkably, such conditioned compensatory responses in turn decrease the impact of the drug on the body—because the body has become more sensitive to pain.
This conditioned compensatory response has many implications. For instance, a drug user will be most “tolerant” to the drug in the presence of cues that have been associated with it (because such cues elicit compensatory responses). As a result, overdose is usually not due to an increase in dosage, but to taking the drug in a new place without the familiar cues—which would have otherwise allowed the user to tolerate the drug (see Siegel, Hinson, Krank, & McCully, 1982). Conditioned compensatory responses (which include heightened pain sensitivity and decreased body temperature, among others) might also cause discomfort, thus motivating the drug user to continue usage of the drug to reduce them. This is one of several ways classical conditioning might be a factor in drug addiction and dependence.
A final effect of classical cues is that they motivate ongoing operant behavior (see Balleine, 2005). For example, if a rat has learned via operant conditioning that pressing a lever will give it a drug, in the presence of cues that signal the “drug is coming soon” (like the sound of the lever squeaking), the rat will work harder to press the lever than if those cues weren’t present (i.e., there is no squeaking lever sound). Similarly, in the presence of food-associated cues (e.g., smells), a rat (or an overeater) will work harder for food. And finally, even in the presence of negative cues (like something that signals fear), a rat, a human, or any other organism will work harder to avoid those situations that might lead to trauma. Classical CSs thus have many effects that can contribute to significant behavioral phenomena.
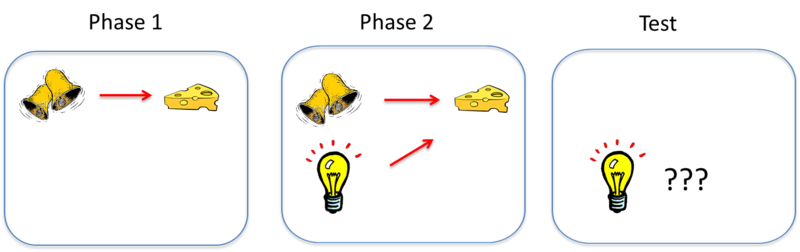
The Learning Process
As mentioned earlier, classical conditioning provides a method for studying basic learning processes. Somewhat counterintuitively, though, studies show that pairing a CS and a US together is not sufficient for an association to be learned between them. Consider an effect called blocking (see Kamin, 1969). In this effect, an animal first learns to associate one CS—call it stimulus A—with a US. In the illustration above, the sound of a bell (stimulus A) is paired with the presentation of food. Once this association is learned, in a second phase, a second stimulus—stimulus B—is presented alongside stimulus A, such that the two stimuli are paired with the US together. In the illustration, a light is added and turned on at the same time the bell is rung. However, because the animal has already learned the association between stimulus A (the bell) and the food, the animal doesn’t learn an association between stimulus B (the light) and the food. That is, the conditioned response only occurs during the presentation of stimulus A, because the earlier conditioning of A “blocks” the conditioning of B when B is added to A. The reason? Stimulus A already predicts the US, so the US is not surprising when it occurs with Stimulus B.
Learning depends on such a surprise, or a discrepancy between what occurs on a conditioning trial and what is already predicted by cues that are present on the trial. To learn something through classical conditioning, there must first be some prediction error, or the chance that a conditioned stimulus won’t lead to the expected outcome. With the example of the bell and the light, because the bell always leads to the reward of food, there’s no “prediction error” that the addition of the light helps to correct. However, if the researcher suddenly requires that the bell and the light both occur in order to receive the food, the bell alone will produce a prediction error that the animal has to learn.
Blocking and other related effects indicate that the learning process tends to take in the most valid predictors of significant events and ignore the less useful ones. This is common in the real world. For example, imagine that your supermarket puts big star-shaped stickers on products that are on sale. Quickly, you learn that items with the big star-shaped stickers are cheaper. However, imagine you go into a similar supermarket that not only uses these stickers, but also uses bright orange price tags to denote a discount. Because of blocking (i.e., you already know that the star-shaped stickers indicate a discount), you don’t have to learn the color system, too. The star-shaped stickers tell you everything you need to know (i.e. there’s no prediction error for the discount), and thus the color system is irrelevant.
Classical conditioning is strongest if the CS and US are intense or salient. It is also best if the CS and US are relatively new and the organism hasn’t been frequently exposed to them before. And it is especially strong if the organism’s biology has prepared it to associate a particular CS and US. For example, rats and humans are naturally inclined to associate an illness with a flavor, rather than with a light or tone. Because foods are most commonly experienced by taste, if there is a particular food that makes us ill, associating the flavor (rather than the appearance—which may be similar to other foods) with the illness will more greatly ensure we avoid that food in the future, and thus avoid getting sick. This sorting tendency, which is set up by evolution, is called preparedness.
There are many factors that affect the strength of classical conditioning, and these have been the subject of much research and theory (see Rescorla & Wagner, 1972; Pearce & Bouton, 2001). Behavioral neuroscientists have also used classical conditioning to investigate many of the basic brain processes that are involved in learning (see Fanselow & Poulos, 2005; Thompson & Steinmetz, 2009).
Erasing Classical Learning
After conditioning, the response to the CS can be eliminated if the CS is presented repeatedly without the US. This effect is called extinction, and the response is said to become “extinguished.” For example, if Pavlov kept ringing the bell but never gave the dog any food afterward, eventually the dog’s CR (drooling) would no longer happen when it heard the CS (the bell), because the bell would no longer be a predictor of food. Extinction is important for many reasons. For one thing, it is the basis for many therapies that clinical psychologists use to eliminate maladaptive and unwanted behaviors. Take the example of a person who has a debilitating fear of spiders: one approach might include systematic exposure to spiders. Whereas, initially the person has a CR (e.g., extreme fear) every time s/he sees the CS (e.g., the spider), after repeatedly being shown pictures of spiders in neutral conditions, pretty soon the CS no longer predicts the CR (i.e., the person doesn’t have the fear reaction when seeing spiders, having learned that spiders no longer serve as a “cue” for that fear). Here, repeated exposure to spiders without an aversive consequence causes extinction.
Psychologists must accept one important fact about extinction, however: it does not necessarily destroy the original learning (see Bouton, 2004). For example, imagine you strongly associate the smell of chalkboards with the agony of middle school detention. Now imagine that, after years of encountering chalkboards, the smell of them no longer recalls the agony of detention (an example of extinction). However, one day, after entering a new building for the first time, you suddenly catch a whiff of a chalkboard and WHAM!, the agony of detention returns. This is called spontaneous recovery: following a lapse in exposure to the CS after extinction has occurred, sometimes re-exposure to the CS (e.g., the smell of chalkboards) can evoke the CR again (e.g., the agony of detention).
Another related phenomenon is the renewal effect: After extinction, if the CS is tested in a new context, such as a different room or location, the CR can also return. In the chalkboard example, the action of entering a new building—where you don’t expect to smell chalkboards—suddenly renews the sensations associated with detention. These effects have been interpreted to suggest that extinction inhibits rather than erases the learned behavior, and this inhibition is mainly expressed in the context in which it is learned (see “context” in the Key Vocabulary section below).
This does not mean that extinction is a bad treatment for behavior disorders. Instead, clinicians can increase its effectiveness by using basic research on learning to help defeat these relapse effects (see Craske et al., 2008). For example, conducting extinction therapies in contexts where patients might be most vulnerable to relapsing (e.g., at work), might be a good strategy for enhancing the therapy’s success.
Useful Things to Know about Instrumental Conditioning
Most of the things that affect the strength of classical conditioning also affect the strength of instrumental learning—whereby we learn to associate our actions with their outcomes. As noted earlier, the “bigger” the reinforcer (or punisher), the stronger the learning. And, if an instrumental behavior is no longer reinforced, it will also be extinguished. Most of the rules of associative learning that apply to classical conditioning also apply to instrumental learning, but other facts about instrumental learning are also worth knowing.
Instrumental Responses Come Under Stimulus Control
As you know, the classic operant response in the laboratory is lever-pressing in rats, reinforced by food. However, things can be arranged so that lever-pressing only produces pellets when a particular stimulus is present. For example, lever-pressing can be reinforced only when a light in the Skinner box is turned on; when the light is off, no food is released from lever-pressing. The rat soon learns to discriminate between the light-on and light-off conditions, and presses the lever only in the presence of the light (responses in light-off are extinguished). In everyday life, think about waiting in the turn lane at a traffic light. Although you know that green means go, only when you have the green arrow do you turn. In this regard, the operant behavior is now said to be under stimulus control. And, as is the case with the traffic light, in the real world, stimulus control is probably the rule.
The stimulus controlling the operant response is called a discriminative stimulus. It can be associated directly with the response, or the reinforcer (see below). However, it usually does not elicit the response the way a classical CS does. Instead, it is said to “set the occasion for” the operant response. For example, a canvas put in front of an artist does not elicit painting behavior or compel her to paint. It allows, or sets the occasion for, painting to occur.
Stimulus-control techniques are widely used in the laboratory to study perception and other psychological processes in animals. For example, the rat would not be able to respond appropriately to light-on and light-off conditions if it could not see the light. Following this logic, experiments using stimulus-control methods have tested how well animals see colors, hear ultrasounds, and detect magnetic fields. That is, researchers pair these discriminative stimuli with those they know the animals already understand (such as pressing the lever). In this way, the researchers can test if the animals can learn to press the lever only when an ultrasound is played, for example.
These methods can also be used to study “higher” cognitive processes. For example, pigeons can learn to peck at different buttons in a Skinner box when pictures of flowers, cars, chairs, or people are shown on a miniature TV screen (see Wasserman, 1995). Pecking button 1 (and no other) is reinforced in the presence of a flower image, button 2 in the presence of a chair image, and so on. Pigeons can learn the discrimination readily, and, under the right conditions, will even peck the correct buttons associated with pictures of new flowers, cars, chairs, and people they have never seen before. The birds have learned to categorize the sets of stimuli. Stimulus-control methods can be used to study how such categorization is learned.
Operant Conditioning Involves Choice
 Another thing to know about operant conditioning is that the response always requires choosing one behavior over others. The student who goes to the bar on Thursday night chooses to drink instead of staying at home and studying. The rat chooses to press the lever instead of sleeping or scratching its ear in the back of the box. The alternative behaviors are each associated with their own reinforcers. And the tendency to perform a particular action depends on both the reinforcers earned for it and the reinforcers earned for its alternatives.
Another thing to know about operant conditioning is that the response always requires choosing one behavior over others. The student who goes to the bar on Thursday night chooses to drink instead of staying at home and studying. The rat chooses to press the lever instead of sleeping or scratching its ear in the back of the box. The alternative behaviors are each associated with their own reinforcers. And the tendency to perform a particular action depends on both the reinforcers earned for it and the reinforcers earned for its alternatives.
To investigate this idea, choice has been studied in the Skinner box by making two levers available for the rat (or two buttons available for the pigeon), each of which has its own reinforcement or payoff rate. A thorough study of choice in situations like this has led to a rule called the quantitative law of effect (see Herrnstein, 1970), which can be understood without going into quantitative detail: The law acknowledges the fact that the effects of reinforcing one behavior depend crucially on how much reinforcement is earned for the behavior’s alternatives. For example, if a pigeon learns that pecking one light will reward two food pellets, whereas the other light only rewards one, the pigeon will only peck the first light. However, what happens if the first light is more strenuous to reach than the second one? Will the cost of energy outweigh the bonus of food? Or will the extra food be worth the work? In general, a given reinforcer will be less reinforcing if there are many alternative reinforcers in the environment. For this reason, alcohol, sex, or drugs may be less powerful reinforcers if the person’s environment is full of other sources of reinforcement, such as achievement at work or love from family members.
Cognition in Instrumental Learning
Modern research also indicates that reinforcers do more than merely strengthen or “stamp in” the behaviors they are a consequence of, as was Thorndike’s original view. Instead, animals learn about the specific consequences of each behavior, and will perform a behavior depending on how much they currently want—or “value”—its consequence.
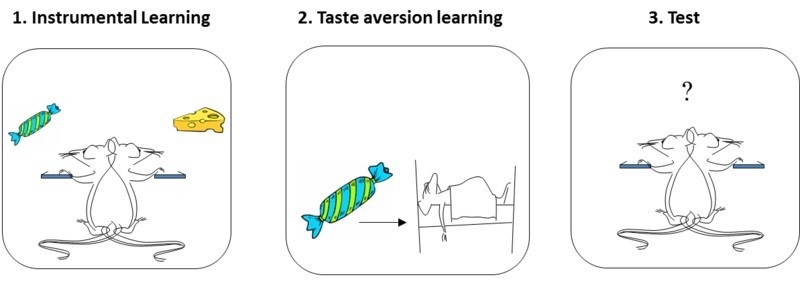
This idea is best illustrated by a phenomenon called the reinforcer devaluation effect (see Colwill & Rescorla, 1986). A rat is first trained to perform two instrumental actions (e.g., pressing a lever on the left, and on the right), each paired with a different reinforcer (e.g., a sweet sucrose solution, and a food pellet). At the end of this training, the rat tends to press both levers, alternating between the sucrose solution and the food pellet. In a second phase, one of the reinforcers (e.g., the sucrose) is then separately paired with illness. This conditions a taste aversion to the sucrose. In a final test, the rat is returned to the Skinner box and allowed to press either lever freely. No reinforcers are presented during this test (i.e., no sucrose or food comes from pressing the levers), so behavior during testing can only result from the rat’s memory of what it has learned earlier. Importantly here, the rat chooses not to perform the response that once produced the reinforcer that it now has an aversion to (e.g., it won’t press the sucrose lever). This means that the rat has learned and remembered the reinforcer associated with each response, and can combine that knowledge with the knowledge that the reinforcer is now “bad.” Reinforcers do not merely stamp in responses; the animal learns much more than that. The behavior is said to be “goal-directed” (see Dickinson & Balleine, 1994), because it is influenced by the current value of its associated goal (i.e., how much the rat wants/doesn’t want the reinforcer).
Things can get more complicated, however, if the rat performs the instrumental actions frequently and repeatedly. That is, if the rat has spent many months learning the value of pressing each of the levers, the act of pressing them becomes automatic and routine. And here, this once goal-directed action (i.e., the rat pressing the lever for the goal of getting sucrose/food) can become a habit. Thus, if a rat spends many months performing the lever-pressing behavior (turning such behavior into a habit), even when sucrose is again paired with illness, the rat will continue to press that lever (see Holland, 2004). After all the practice, the instrumental response (pressing the lever) is no longer sensitive to reinforcer devaluation. The rat continues to respond automatically, regardless of the fact that the sucrose from this lever makes it sick.
Habits are very common in human experience, and can be useful. You do not need to relearn each day how to make your coffee in the morning or how to brush your teeth. Instrumental behaviors can eventually become habitual, letting us get the job done while being free to think about other things.
Putting Classical and Instrumental Conditioning Together
Classical and operant conditioning are usually studied separately. But outside of the laboratory they almost always occur at the same time. For example, a person who is reinforced for drinking alcohol or eating excessively learns these behaviors in the presence of certain stimuli—a pub, a set of friends, a restaurant, or possibly the couch in front of the TV. These stimuli are also available for association with the reinforcer. In this way, classical and operant conditioning are always intertwined.
The figure below summarizes this idea, and helps review what we have discussed in this module. Generally speaking, any reinforced or punished operant response (R) is paired with an outcome (O) in the presence of some stimulus or set of stimuli (S).
The figure illustrates the types of associations that can be learned in this very general scenario. For one thing, the organism will learn to associate the response and the outcome (R – O). This is instrumental conditioning. The learning process here is probably similar to classical conditioning, with all its emphasis on surprise and prediction error. And, as we discussed while considering the reinforcer devaluation effect, once R – O is learned, the organism will be ready to perform the response if the outcome is desired or valued. The value of the reinforcer can also be influenced by other reinforcers earned for other behaviors in the situation. These factors are at the heart of instrumental learning.
Second, the organism can also learn to associate the stimulus with the reinforcing outcome (S – O). This is the classical conditioning component, and as we have seen, it can have many consequences on behavior. For one thing, the stimulus will come to evoke a system of responses that help the organism prepare for the reinforcer (not shown in the figure): The drinker may undergo changes in body temperature; the eater may salivate and have an increase in insulin secretion. In addition, the stimulus will evoke approach (if the outcome is positive) or retreat (if the outcome is negative). Presenting the stimulus will also prompt the instrumental response.
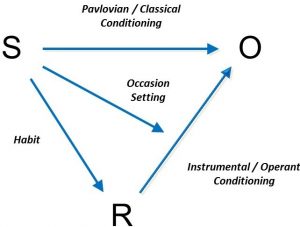 The third association in the diagram is the one between the stimulus and the response (S – R). As discussed earlier, after a lot of practice, the stimulus may begin to elicit the response directly. This is habit learning, whereby the response occurs relatively automatically, without much mental processing of the relation between the action and the outcome and the outcome’s current value.
The third association in the diagram is the one between the stimulus and the response (S – R). As discussed earlier, after a lot of practice, the stimulus may begin to elicit the response directly. This is habit learning, whereby the response occurs relatively automatically, without much mental processing of the relation between the action and the outcome and the outcome’s current value.
The final link in the figure is between the stimulus and the response-outcome association [S – (R – O)]. More than just entering into a simple association with the R or the O, the stimulus can signal that the R – O relationship is now in effect. This is what we mean when we say that the stimulus can “set the occasion” for the operant response: It sets the occasion for the response-reinforcer relationship. Through this mechanism, the painter might begin to paint when given the right tools and the opportunity enabled by the canvas. The canvas theoretically signals that the behavior of painting will now be reinforced by positive consequences.
The figure provides a framework that you can use to understand almost any learned behavior you observe in yourself, your family, or your friends. If you would like to understand it more deeply, consider taking a course on learning in the future, which will give you a fuller appreciation of how classical learning, instrumental learning, habit learning, and occasion setting actually work and interact.
Observational Learning
Not all forms of learning are accounted for entirely by classical and operant conditioning. Imagine a child walking up to a group of children playing a game on the playground. The game looks fun, but it is new and unfamiliar. Rather than joining the game immediately, the child opts to sit back and watch the other children play a round or two. Observing the others, the child takes note of the ways in which they behave while playing the game. By watching the behavior of the other kids, the child can figure out the rules of the game and even some strategies for doing well at the game. This is called observational learning.

Observational learning is a component of Albert Bandura’s Social Learning Theory (Bandura, 1977), which posits that individuals can learn novel responses via observation of key others’ behaviors. Observational learning does not necessarily require reinforcement, but instead hinges on the presence of others, referred to as social models. Social models are typically of higher status or authority compared to the observer, examples of which include parents, teachers, and police officers. In the example above, the children who already know how to play the game could be thought of as being authorities—and are therefore social models—even though they are the same age as the observer. By observing how the social models behave, an individual is able to learn how to act in a certain situation. Other examples of observational learning might include a child learning to place her napkin in her lap by watching her parents at the dinner table, or a customer learning where to find the ketchup and mustard after observing other customers at a hot dog stand.
Bandura theorizes that the observational learning process consists of four parts. The first is attention—as, quite simply, one must pay attention to what s/he is observing in order to learn. The second part is retention: to learn one must be able to retain the behavior s/he is observing in memory. The third part of observational learning, initiation, acknowledges that the learner must be able to execute (or initiate) the learned behavior. Lastly, the observer must possess the motivation to engage in observational learning. In our vignette, the child must want to learn how to play the game in order to properly engage in observational learning.
Researchers have conducted countless experiments designed to explore observational learning, the most famous of which is Albert Bandura’s “Bobo doll experiment.”
In this experiment (Bandura, Ross & Ross 1961), Bandura had children individually observe an adult social model interact with a clown doll (“Bobo”). For one group of children, the adult interacted aggressively with Bobo: punching it, kicking it, throwing it, and even hitting it in the face with a toy mallet. Another group of children watched the adult interact with other toys, displaying no aggression toward Bobo. In both instances the adult left and the children were allowed to interact with Bobo on their own. Bandura found that children exposed to the aggressive social model were significantly more likely to behave aggressively toward Bobo, hitting and kicking him, compared to those exposed to the non-aggressive model. The researchers concluded that the children in the aggressive group used their observations of the adult social model’s behavior to determine that aggressive behavior toward Bobo was acceptable.
While reinforcement was not required to elicit the children’s behavior in Bandura’s first experiment, it is important to acknowledge that consequences do play a role within observational learning. A future adaptation of this study (Bandura, Ross, & Ross, 1963) demonstrated that children in the aggression group showed less aggressive behavior if they witnessed the adult model receive punishment for aggressing against Bobo. Bandura referred to this process as vicarious reinforcement, as the children did not experience the reinforcement or punishment directly, yet were still influenced by observing it.
Comparative Cognition at Queen’s University
Queen’s Psychology offers a course for those interested in learning more about the science of learning, and about similarities and differences of cognition across animal species (including humans!). If you are interested in learning more about this area, we encourage you to consider PSYC305: Comparative Cognition.
Conclusion
We have covered three primary explanations for how we learn to behave and interact with the world around us. Considering your own experiences, how well do these theories apply to you? Maybe when reflecting on your personal sense of fashion, you realize that you tend to select clothes others have complimented you on (operant conditioning). Or maybe, thinking back on a new restaurant you tried recently, you realize you chose it because its commercials play happy music (classical conditioning). Or maybe you are now always on time with your assignments, because you saw how others were punished when they were late (observational learning). Regardless of the activity, behavior, or response, there’s a good chance your “decision” to do it can be explained based on one of the theories presented in this module.
Check Your Knowledge
To help you with your studying, we’ve included some practice questions for this module. These questions do not necessarily address all content in this module. They are intended as practice, and you are responsible for all of the content in this module even if there is no associated practice question. To promote deeper engagement with the material, we encourage you to create some questions of your own for your practice. You can then also return to these self-generated questions later in the course to test yourself.
Vocabulary
- Blocking
- In classical conditioning, the finding that no conditioning occurs to a stimulus if it is combined with a previously conditioned stimulus during conditioning trials. Suggests that information, surprise value, or prediction error is important in conditioning.
- Categorize
- To sort or arrange different items into classes or categories.
- Classical conditioning
- The procedure in which an initially neutral stimulus (the conditioned stimulus, or CS) is paired with an unconditioned stimulus (or US). The result is that the conditioned stimulus begins to elicit a conditioned response (CR). Classical conditioning is nowadays considered important as both a behavioral phenomenon and as a method to study simple associative learning. Same as Pavlovian conditioning.
- Conditioned compensatory response
- In classical conditioning, a conditioned response that opposes, rather than is the same as, the unconditioned response. It functions to reduce the strength of the unconditioned response. Often seen in conditioning when drugs are used as unconditioned stimuli.
- Conditioned response (CR)
- The response that is elicited by the conditioned stimulus after classical conditioning has taken place.
- Conditioned stimulus (CS)
- An initially neutral stimulus (like a bell, light, or tone) that elicits a conditioned response after it has been associated with an unconditioned stimulus.
- Context
- Stimuli that are in the background whenever learning occurs. For instance, the Skinner box or room in which learning takes place is the classic example of a context. However, “context” can also be provided by internal stimuli, such as the sensory effects of drugs (e.g., being under the influence of alcohol has stimulus properties that provide a context) and mood states (e.g., being happy or sad). It can also be provided by a specific period in time—the passage of time is sometimes said to change the “temporal context.”
- Discriminative stimulus
- In operant conditioning, a stimulus that signals whether the response will be reinforced. It is said to “set the occasion” for the operant response.
- Extinction
- Decrease in the strength of a learned behavior that occurs when the conditioned stimulus is presented without the unconditioned stimulus (in classical conditioning) or when the behavior is no longer reinforced (in instrumental conditioning). The term describes both the procedure (the US or reinforcer is no longer presented) as well as the result of the procedure (the learned response declines). Behaviors that have been reduced in strength through extinction are said to be “extinguished.”
- Fear conditioning
- A type of classical or Pavlovian conditioning in which the conditioned stimulus (CS) is associated with an aversive unconditioned stimulus (US), such as a foot shock. As a consequence of learning, the CS comes to evoke fear. The phenomenon is thought to be involved in the development of anxiety disorders in humans.
- Goal-directed behavior
- Instrumental behavior that is influenced by the animal’s knowledge of the association between the behavior and its consequence and the current value of the consequence. Sensitive to the reinforcer devaluation effect.
- Habit
- Instrumental behavior that occurs automatically in the presence of a stimulus and is no longer influenced by the animal’s knowledge of the value of the reinforcer. Insensitive to the reinforcer devaluation effect.
- Instrumental conditioning
- Process in which animals learn about the relationship between their behaviors and their consequences. Also known as operant conditioning.
- Law of effect
- The idea that instrumental or operant responses are influenced by their effects. Responses that are followed by a pleasant state of affairs will be strengthened and those that are followed by discomfort will be weakened. Nowadays, the term refers to the idea that operant or instrumental behaviors are lawfully controlled by their consequences.
- Observational learning
- Learning by observing the behavior of others.
- Operant
- A behavior that is controlled by its consequences. The simplest example is the rat’s lever-pressing, which is controlled by the presentation of the reinforcer.
- Operant conditioning
- See instrumental conditioning.
- Pavlovian conditioning
- See classical conditioning.
- Prediction error
- When the outcome of a conditioning trial is different from that which is predicted by the conditioned stimuli that are present on the trial (i.e., when the US is surprising). Prediction error is necessary to create Pavlovian conditioning (and associative learning generally). As learning occurs over repeated conditioning trials, the conditioned stimulus increasingly predicts the unconditioned stimulus, and prediction error declines. Conditioning works to correct or reduce prediction error.
- Preparedness
- The idea that an organism’s evolutionary history can make it easy to learn a particular association. Because of preparedness, you are more likely to associate the taste of tequila, and not the circumstances surrounding drinking it, with getting sick. Similarly, humans are more likely to associate images of spiders and snakes than flowers and mushrooms with aversive outcomes like shocks.
- Punisher
- A stimulus that decreases the strength of an operant behavior when it is made a consequence of the behavior.
- Quantitative law of effect
- A mathematical rule that states that the effectiveness of a reinforcer at strengthening an operant response depends on the amount of reinforcement earned for all alternative behaviors. A reinforcer is less effective if there is a lot of reinforcement in the environment for other behaviors.
- Reinforcer
- Any consequence of a behavior that strengthens the behavior or increases the likelihood that it will be performed it again.
- Reinforcer devaluation effect
- The finding that an animal will stop performing an instrumental response that once led to a reinforcer if the reinforcer is separately made aversive or undesirable.
- Renewal effect
- Recovery of an extinguished response that occurs when the context is changed after extinction. Especially strong when the change of context involves return to the context in which conditioning originally occurred. Can occur after extinction in either classical or instrumental conditioning.
- The theory that people can learn new responses and behaviors by observing the behavior of others.
- Authorities that are the targets for observation and who model behaviors.
- Spontaneous recovery
- Recovery of an extinguished response that occurs with the passage of time after extinction. Can occur after extinction in either classical or instrumental conditioning.
- Stimulus control
- When an operant behavior is controlled by a stimulus that precedes it.
- Taste aversion learning
- The phenomenon in which a taste is paired with sickness, and this causes the organism to reject—and dislike—that taste in the future.
- Unconditioned response (UR)
- In classical conditioning, an innate response that is elicited by a stimulus before (or in the absence of) conditioning.
- Unconditioned stimulus (US)
- In classical conditioning, the stimulus that elicits the response before conditioning occurs.
- Vicarious reinforcement
- Learning that occurs by observing the reinforcement or punishment of another person.
References
- Balleine, B. W. (2005). Neural basis of food-seeking: Affect, arousal, and reward in corticostratolimbic circuits. Physiology & Behavior, 86, 717–730.
- Bandura, A. (1977). Social learning theory. Englewood Cliffs, NJ: Prentice Hall
- Bandura, A., Ross, D., Ross, S (1963). Imitation of film-mediated aggressive models. Journal of Abnormal and Social Psychology 66(1), 3 – 11.
- Bandura, A.; Ross, D.; Ross, S. A. (1961). “Transmission of aggression through the imitation of aggressive models”. Journal of Abnormal and Social Psychology 63(3), 575–582.
- Bernstein, I. L. (1991). Aversion conditioning in response to cancer and cancer treatment. Clinical Psychology Review, 11, 185–191.
- Bouton, M. E. (2004). Context and behavioral processes in extinction. Learning & Memory, 11, 485–494.
- Colwill, R. M., & Rescorla, R. A. (1986). Associative structures in instrumental learning. In G. H. Bower (Ed.), The psychology of learning and motivation, (Vol. 20, pp. 55–104). New York, NY: Academic Press.
- Craske, M. G., Kircanski, K., Zelikowsky, M., Mystkowski, J., Chowdhury, N., & Baker, A. (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46, 5–27.
- Dickinson, A., & Balleine, B. W. (1994). Motivational control of goal-directed behavior. Animal Learning & Behavior, 22, 1–18.
- Fanselow, M. S., & Poulos, A. M. (2005). The neuroscience of mammalian associative learning. Annual Review of Psychology, 56, 207–234.
- Herrnstein, R. J. (1970). On the law of effect. Journal of the Experimental Analysis of Behavior, 13, 243–266.
- Holland, P. C. (2004). Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes, 30, 104–117.
- Kamin, L. J. (1969). Predictability, surprise, attention, and conditioning. In B. A. Campbell & R. M. Church (Eds.), Punishment and aversive behavior (pp. 279–296). New York, NY: Appleton-Century-Crofts.
- Mineka, S., & Zinbarg, R. (2006). A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist, 61, 10–26.
- Pearce, J. M., & Bouton, M. E. (2001). Theories of associative learning in animals. Annual Review of Psychology, 52, 111–139.
- Rescorla, R. A., & Wagner, A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). New York, NY: Appleton-Century-Crofts.
- Scalera, G., & Bavieri, M. (2009). Role of conditioned taste aversion on the side effects of chemotherapy in cancer patients. In S. Reilly & T. R. Schachtman (Eds.), Conditioned taste aversion: Behavioral and neural processes (pp. 513–541). New York, NY: Oxford University Press.
- Siegel, S. (1989). Pharmacological conditioning and drug effects. In A. J. Goudie & M. Emmett-Oglesby (Eds.), Psychoactive drugs (pp. 115–180). Clifton, NY: Humana Press.
- Siegel, S., Hinson, R. E., Krank, M. D., & McCully, J. (1982). Heroin “overdose” death: Contribution of drug associated environmental cues. Science, 216, 436–437.
- Spreat, S., & Spreat, S. R. (1982). Learning principles. In V. Voith & P. L. Borchelt (Eds.), Veterinary clinics of North America: Small animal practice (pp. 593–606). Philadelphia, PA: W. B. Saunders.
- Thompson, R. F., & Steinmetz, J. E. (2009). The role of the cerebellum in classical conditioningof discrete behavioral responses. Neuroscience, 162, 732–755.
- Timberlake, W. L. (2001). Motivational modes in behavior systems. In R. R. Mowrer & S. B. Klein (Eds.), Handbook of contemporary learning theories (pp. 155–210). Mahwah, NJ: Lawrence Erlbaum Associates, Inc.
- Wasserman, E. A. (1995). The conceptual abilities of pigeons. American Scientist, 83, 246–255.
How to cite this Chapter using APA Style:
Bouton, M. E. (2019). Conditioning and learning. Adapted for use by Queen’s University. Original chapter in R. Biswas-Diener & E. Diener (Eds), Noba textbook series: Psychology. Champaign, IL: DEF publishers. Retrieved from http://noba.to/ajxhcqdr
Copyright and Acknowledgment:
This material is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit: http://creativecommons.org/licenses/by-nc-sa/4.0/deed.en_US.
This material is attributed to the Diener Education Fund (copyright © 2018) and can be accessed via this link: http://noba.to/ajxhcqdr.
Additional information about the Diener Education Fund (DEF) can be accessed here.
Describes stimulus-stimulus associative learning.
See classical conditioning.
In classical conditioning, the stimulus that elicits the response before conditioning occurs.
In classical conditioning, an innate response that is elicited by a stimulus before (or in the absence of) conditioning.
An initially neutral stimulus (like a bell, light, or tone) that elicits a conditioned response after it has been associated with an unconditioned stimulus.
The response that is elicited by the conditioned stimulus after classical conditioning has taken place.
Process in which animals learn about the relationship between their behaviors and their consequences. Also known as operant conditioning.
Describes stimulus-response associative learning.
A behavior that is controlled by its consequences. The simplest example is the rat’s lever-pressing, which is controlled by the presentation of the reinforcer.
Any consequence of a behavior that strengthens the behavior or increases the likelihood that it will be performed it again.
The idea that instrumental or operant responses are influenced by their effects. Responses that are followed by a pleasant state of affairs will be strengthened and those that are followed by discomfort will be weakened. Nowadays, the term refers to the idea that operant or instrumental behaviors are lawfully controlled by their consequences.
A stimulus that decreases the strength of an operant behavior when it is made a consequence of the behavior.
Spreat, S., & Spreat, S. R. (1982). Learning principles. In V. Voith & P. L. Borchelt (Eds.), Veterinary clinics of North America: Small animal practice (pp. 593–606). Philadelphia, PA: W. B. Saunders.
Timberlake, W. L. (2001). Motivational modes in behavior systems. In R. R. Mowrer & S. B. Klein (Eds.), Handbook of contemporary learning theories (pp. 155–210). Mahwah, NJ: Lawrence Erlbaum Associates, Inc.
The phenomenon in which a taste is paired with sickness, and this causes the organism to reject—and dislike—that taste in the future.
Bernstein, I. L. (1991). Aversion conditioning in response to cancer and cancer treatment. Clinical Psychology Review, 11, 185–191.
Scalera, G., & Bavieri, M. (2009). Role of conditioned taste aversion on the side effects of chemotherapy in cancer patients. In S. Reilly & T. R. Schachtman (Eds.), Conditioned taste aversion: Behavioral and neural processes (pp. 513–541). New York, NY: Oxford University Press.
A type of classical or Pavlovian conditioning in which the conditioned stimulus (CS) is associated with an aversive unconditioned stimulus (US), such as a foot shock. As a consequence of learning, the CS comes to evoke fear. The phenomenon is thought to be involved in the development of anxiety disorders in humans.
Mineka, S., & Zinbarg, R. (2006). A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. American Psychologist, 61, 10–26.
Siegel, S. (1989). Pharmacological conditioning and drug effects. In A. J. Goudie & M. Emmett-Oglesby (Eds.), Psychoactive drugs (pp. 115–180). Clifton, NY: Humana Press.
In classical conditioning, a conditioned response that opposes, rather than is the same as, the unconditioned response. It functions to reduce the strength of the unconditioned response. Often seen in conditioning when drugs are used as unconditioned stimuli.
Siegel, S., Hinson, R. E., Krank, M. D., & McCully, J. (1982). Heroin “overdose” death: Contribution of drug associated environmental cues. Science, 216, 436–437.
Balleine, B. W. (2005). Neural basis of food-seeking: Affect, arousal, and reward in corticostratolimbic circuits. Physiology & Behavior, 86, 717–730.
In classical conditioning, the finding that no conditioning occurs to a stimulus if it is combined with a previously conditioned stimulus during conditioning trials. Suggests that information, surprise value, or prediction error is important in conditioning.
Kamin, L. J. (1969). Predictability, surprise, attention, and conditioning. In B. A. Campbell & R. M. Church (Eds.), Punishment and aversive behavior (pp. 279–296). New York, NY: Appleton-Century-Crofts.
When the outcome of a conditioning trial is different from that which is predicted by the conditioned stimuli that are present on the trial (i.e., when the US is surprising). Prediction error is necessary to create Pavlovian conditioning (and associative learning generally). As learning occurs over repeated conditioning trials, the conditioned stimulus increasingly predicts the unconditioned stimulus, and prediction error declines. Conditioning works to correct or reduce prediction error.
The idea that an organism’s evolutionary history can make it easy to learn a particular association. Because of preparedness, you are more likely to associate the taste of tequila, and not the circumstances surrounding drinking it, with getting sick. Similarly, humans are more likely to associate images of spiders and snakes than flowers and mushrooms with aversive outcomes like shocks.
Rescorla, R. A., & Wagner, A. R. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). New York, NY: Appleton-Century-Crofts.
Pearce, J. M., & Bouton, M. E. (2001). Theories of associative learning in animals. Annual Review of Psychology, 52, 111–139.
Fanselow, M. S., & Poulos, A. M. (2005). The neuroscience of mammalian associative learning. Annual Review of Psychology, 56, 207–234.
Thompson, R. F., & Steinmetz, J. E. (2009). The role of the cerebellum in classical conditioningof discrete behavioral responses. Neuroscience, 162, 732–755.
Decrease in the strength of a learned behavior that occurs when the conditioned stimulus is presented without the unconditioned stimulus (in classical conditioning) or when the behavior is no longer reinforced (in instrumental conditioning). The term describes both the procedure (the US or reinforcer is no longer presented) as well as the result of the procedure (the learned response declines). Behaviors that have been reduced in strength through extinction are said to be “extinguished.”
Bouton, M. E. (2004). Context and behavioral processes in extinction. Learning & Memory, 11, 485–494.
Recovery of an extinguished response that occurs with the passage of time after extinction. Can occur after extinction in either classical or instrumental conditioning.
Recovery of an extinguished response that occurs when the context is changed after extinction. Especially strong when the change of context involves return to the context in which conditioning originally occurred. Can occur after extinction in either classical or instrumental conditioning.
Stimuli that are in the background whenever learning occurs. For instance, the Skinner box or room in which learning takes place is the classic example of a context. However, “context” can also be provided by internal stimuli, such as the sensory effects of drugs (e.g., being under the influence of alcohol has stimulus properties that provide a context) and mood states (e.g., being happy or sad). It can also be provided by a specific period in time—the passage of time is sometimes said to change the “temporal context.”
Craske, M. G., Kircanski, K., Zelikowsky, M., Mystkowski, J., Chowdhury, N., & Baker, A. (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy, 46, 5–27.
When an operant behavior is controlled by a stimulus that precedes it.
In operant conditioning, a stimulus that signals whether the response will be reinforced. It is said to “set the occasion” for the operant response.
Wasserman, E. A. (1995). The conceptual abilities of pigeons. American Scientist, 83, 246–255.
To sort or arrange different items into classes or categories.
A mathematical rule that states that the effectiveness of a reinforcer at strengthening an operant response depends on the amount of reinforcement earned for all alternative behaviors. A reinforcer is less effective if there is a lot of reinforcement in the environment for other behaviors.
Herrnstein, R. J. (1970). On the law of effect. Journal of the Experimental Analysis of Behavior, 13, 243–266.
The finding that an animal will stop performing an instrumental response that once led to a reinforcer if the reinforcer is separately made aversive or undesirable.
Colwill, R. M., & Rescorla, R. A. (1986). Associative structures in instrumental learning. In G. H. Bower (Ed.), The psychology of learning and motivation, (Vol. 20, pp. 55–104). New York, NY: Academic Press.
Instrumental behavior that is influenced by the animal’s knowledge of the association between the behavior and its consequence and the current value of the consequence. Sensitive to the reinforcer devaluation effect.
Dickinson, A., & Balleine, B. W. (1994). Motivational control of goal-directed behavior. Animal Learning & Behavior, 22, 1–18.
Instrumental behavior that occurs automatically in the presence of a stimulus and is no longer influenced by the animal’s knowledge of the value of the reinforcer. Insensitive to the reinforcer devaluation effect.
Holland, P. C. (2004). Relations between Pavlovian-instrumental transfer and reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes, 30, 104–117.
Learning by observing the behavior of others.
The theory that people can learn new responses and behaviors by observing the behavior of others.
Bandura, A. (1977). Social learning theory. Englewood Cliffs, NJ: Prentice Hall
Authorities that are the targets for observation and who model behaviors.
Bandura, A.; Ross, D.; Ross, S. A. (1961). “Transmission of aggression through the imitation of aggressive models”. Journal of Abnormal and Social Psychology 63(3), 575–582.
Bandura, A., Ross, D., Ross, S (1963). Imitation of film-mediated aggressive models. Journal of Abnormal and Social Psychology 66(1), 3 – 11.
Learning that occurs by observing the reinforcement or punishment of another person.

