4.5 Neuroscience and Art
Amna Noor and Rena Patricia Seeger
Music
Music is one of the most abstract forms of art and yet can evoke real and measurable physiological mechanisms in the brain, allowing one to feel a part of a different dynamic — a purely musical dynamic (Walton, 1988; Trimble & Hesdorffer, 2017). The music-neuroscience interface has been studied by various researchers recently and in this section, some of the important findings, questions, and controversies will be discussed.
How is Music Processed?
It is important to learn how our brains process music to be able to implement music therapies and make informed choices for various brain traumas and neurological disorders. There is an inherent difficulty in the understanding of music processing due to the sheer plethora of music genres and the different neurological cascades they can kickstart by evoking different emotions such as stress, joy, happiness, melancholy, et cetera (Hernandez-Ruiz, 2019; Tai & Kuo, 2019). Each musical melody is made up of different elements which can be randomly categorized under pitch, tempo and rhythm, volume, timbre, texture, duration, and form. Out of these, only pitch and tempo and rhythm have been studied in detail (Hernandez-Ruiz, 2019).
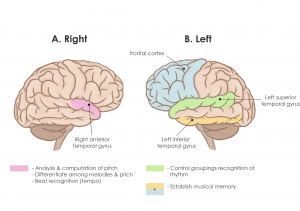
Pitch is analyzed and computed in the right anterior superior temporal gyrus (STG) (Figure 1A). Changes in pitch can activate the posterior secondary auditory cortex. The right temporal cortex can also differentiate among melodies in the presence of contour information or pitch direction. The right anterior STG is also responsible for beat recognition (tempo) while the left STG controls groupings recognition (rhythm). This motor and perceptual timing also involve the functions of the cerebellum, basal ganglia, supplemental motor cortex, premotor cortex, and the parietal cortex (Peretz & Zatorre, 2005; Hernandez-Ruiz, 2019). As shown in Figure 1B, higher-order processing that involves the inferior temporal and frontal cortex is required for the establishment of musical memory (Peretz & Zatorre, 2005).
Overall, it seems that different components of a melody are processed by different areas of the brain. A study compared a musician with Alzheimer’s disease to a musician with semantic dementia to evaluate their abilities to recognize compositions. Musical knowledge was preserved in the musician with semantic dementia even though he experienced a loss of both normal expressive and normal verbal functions. This alludes to the double dissociation theory that suggests musical elements are processed independently and not sequentially (Omar et al., 2010; Hernandez-Ruiz, 2019).
Music Therapy in Neuroscience

Music therapy, defined as “clinical, evidence-based use of music interventions to accomplish individualized goals within a therapeutic relationship by a credentialed professional who has completed an approved music therapy program” is being increasingly used to enhance neural executive functions and neuroplasticity in recent years (American Music Therapy Association, 2018). Taking part in a musical performance, or listening to complex music makes use of multiple parts of the brain which is beneficial for the brain as it gives the brain a chance to enhance dendritic sprouting and promote synaptic plasticity (Vik et al., 2019). This chapter will discuss some of the neuroscience fields benefitting from the therapy.
Neonatal Brain Development
Many epigenetic factors contribute to neonatal brain development such as auditory, visual, or somatosensory factors (Galvan, 2018). Fetus’s auditory system develops around the 16th week of gestation. Following the development, the fetus can detect and process sound stimuli between the 26th and 30th weeks of gestation; this period is considered critical for neurodevelopment. Research has shown that a cacophony of loud people’s voices or lack thereof in the NICU can have detrimental effects on neonatal neurodevelopment which can manifest themselves as delayed language capacity in later years (Loewy & Jaschke, 2020).
A study by Loewy et al. in 2013 demonstrated improvement in vital signs, sleep patterns, and heart rate patterns of infants born prematurely when exposed to either parental or music therapist singing conditions (Loewy & Jaschke, 2020). Studies such as the one mentioned are common but the longterm transfer of these improvements as well as longitudinal studies that record the impact of early music therapy on different cognitive activities are infrequent. However, a study by Hennessy et al. in 2019 suggested that music-based training can play a role in expediting the development of inhibitory controls and associated neural pathways well over childhood (Hennessy et al., 2019).
It is also important to consider different musical parameters when discussing neonatal development, as we have discussed that different musical elements are processed individually before. Recently, a study highlighted the importance of timing of music therapy. Listening to ~8 minutes of music 5 times per week at times of feeding, alertness, or sleepiness before 32 weeks of gestation is associated with a functional brain that is similar in architecture to that of a full-term newborn (Loewy & Jaschke, 2020). Sleep regulation is tightly linked with a strong neurological function. A study explored the relationship of timbre and function of brain regions in the frontal lobe, the thalamus, the hippocampus, the amygdala, planum temporal, and the temporal plane and discovered a positive neurobehavioral development correlation between the two. The study made use of a breathing bear contraception that mimicked breathing babies in the vicinity by making an “ah” sound. Infants that received the device had better respiratory patterns than those who did not and eventually had a quieter and calmer sleep at ~45 weeks (Loewy & Jaschke, 2020; Ingersoll & Thoman, 1994). Other factors that are involved in better development of neural structures and associations in neonates include music that is predictable and familiar (Loewy & Jaschke, 2020).
Traumatic Brain Injury and Neurological Disorders
Traumatic brain injury (TBI) is associated with lifelong impairment of cognitive functions, especially executive functions. Music-supported therapy can improve cognitive functions, particularly in the case of a mild injury by enhancing neuroplasticity — the ability of the brain to form new connections (Siponkoski et al., 2020). TBI usually involves damage to either the orbitofrontal cortex alone or combined with temporal pole damage (Vik et al., 2019). Both of these scenarios have great implications for behaviour because the orbitofrontal cortex is an integration centre for emotional processing (Vik et al., 2019).
A study by Vik et al. trained victims of a TBI to play the piano. They found an enhancement of synaptic connections in regions of the brain that process episodic and semantic memory networks. Elements of repetition can also strengthen the newly established connections, as is observed in learning. Furthermore, during the training the ‘goal’ for the patients was to learn to play the piano when this was achieved, dopamine was released (sense of reward is involved in the release of dopamine). Dopamine release causes the limbic system, the amygdala, and association cortices to be activated and form new connections. Their last finding indicated that post-music intervention, patients were more likely to engage in a social situation rather than withdraw, which indicated an improvement in the orbitofrontal cortex and rostral anterior cingulate gyrus and solidified the use of music therapy as a successful therapy (Vik et al., 2019). Similar results were found in a study that investigated the impact of music therapy on patients with mild TBI (Vik et al., 2018).
Autism Spectrum Disorder (ASD) is characterized by avoidance of social interaction and “repetitive patterns of behaviour, interests or activities” and is linked to deficits and impairments in frontotemporal and frontoparietal regions, the amygdala-hippocampal complex, cerebellum, basal ganglia, and anterior and posterior cingulate regions (Brancatisano et al., 2020). It was found that children suffering from ASD are more likely to respond to musical stimuli rather than normal, verbal stimuli and can focus on musical stimuli longer than controls. The finding has favourable effects on attention, memory, and social interactions for children with ASD (Brancatisano et al., 2020). Music therapy inventions have also had valuable results for patients suffering from dementia, stroke, and Parkinson’s disease (Brancatisano et al., 2020).
Limitations and Conclusions
Although many studies have tried to establish a causative link between music-supported therapy and enhancement of neural connections, the number of long-term studies is minimal, and some studies are not replicable. These limitations can potentially overestimate the effect of music therapy, especially over the years. Furthermore, the studies that use behavioural testing and assume a connection to a particular region of the brain grossly underestimate the complexity of neural pathways which can be activated by a multitude of confounding factors such as mood, environment, and expertise (Hernandez-Ruiz, 2019).
In conclusion, music-supported therapy is an emerging topic in neuroscience that still has a long way to go. The interdisciplinary nature of the music and its effect makes it a difficult topic to study. However, a mechanistic understanding of how all the different elements of music are processed and analyzed by the brain can lead us to the ultimate therapeutic uses of music as a neurological therapy.
Dance
Dance, defined as a choreographed routine of movements usually performed to music (Hui et al., 2009) is one of the more synchronized activities that human body can perform – by involving perceiving and performing rhythm, it is similar to music, however, dance also can convey ideas in ways that are analogous to language. Unlike most of our daily activities, dance integrates brain functions involved in kinesthesia, musicality, and emotion (Teixeira-Machado, Arida, de Jesus Mari, 2019). In this section, we will be exploring how dance affects neuroplasticity, and how dance therapy can be used for neurodegenerative disease.
Neuroplasticity and Dance
Due to its integrative nature, dance is believed to provide a unique model for studying the way the human brain integrates both movement and sound, and how motor experience can be influenced by creativity and performance. A systematic review by Teixeria-Machado, Arida, and de Jesus Mari from 2019 looked at eight studies that explored the influence of dance practice on neuroplasticity in mature brains. All studies demonstrated positive structural and/or functional changes, including increased hippocampal volume, increased gray matter volume in areas like the left precentral and parahippocampal gyrus, as well as significant improvements in memory, attention, and psychosocial parameters. Other studies have shown the impact of dance on the human brain even in the short-term. A study by Kulinna et al. from 2018, looking at the effects of dance on elementary school students, demonstrated that an acute bout of aerobic dance was able to significantly improve students’ processing speed and concentration performance in a selective attention test.
Dance As Treatment
In recent years, there has been an increasing interest in using dance as a form of therapeutic intervention to treat various developmental disorders such as Down syndrome, neurological disorders such as schizophrenia, mood disorders like depression, neuromotor disorders like Parkinson’s disease, and even dementia (Burzynska, Finc, Taylor, Knecht, Kramer, 2017). As dance typically involves motor, cognitive, visuospatial, social, and emotional engagement, compared to other forms of treatment that may only target physical fitness or cognitive abilities, dance is seen as a more holistic form of treatment that is easily accessible and enjoyable.
Age-related changes, including volume reductions in brain areas such as the prefrontal and temporal cortices are primarily due to the shrinking of cells and reduced synaptic density (Rehfeld et al., 2018). With increasing research suggesting that the adult brain is capable of neuroplasticity, there has been an increased interest in discovering new treatments that can counteract these detrimental effects of ageing on the brain.
Animal research has demonstrated that only a combination of physical activity and sensory enrichment can have lasting impacts on adult neuroplasticity. Dance is thus seen as an appealing intervention as it demands both physical and cognitive functions. When comparing dance therapy to traditional forms of exercise interventions, although both are associated with increases in physical fitness levels, individuals who had undergone dance therapy saw larger volume increases in brain areas such as the cingulate cortex, insula, corpus callosum, and sensorimotor cortex (Rehfeld et al., 2018). A study by Rehfeld et al. from 2018 found that participants who had undergone a dance program, and not the conventional fitness program, showed increases in plasma BDNF levels. BDNF is an important growth factor supporting functions such as synaptic plasticity in the CNS.
Parkinson’s Disorder and Dance
Parkinson’s disease is a neurodegenerative disorder characterized by motor impairments such as bradykinesia, rigidity, resting tremor and gait problems, as well as non-motor symptoms including sleep disorders, sensory alteration, cognitive impairment and depression (Nemes et al., 2019). Although often classified as a ‘motor disorder’, its effects on psychological, emotional, social and financial functions of life are often what make PD so debilitating (Sharp and Hewitt, 2014). As a result, there is a strong need for interventions that cater towards treating not just the motor symptoms, but other aspects of quality of life.
There is accumulating research suggesting that regular physical activity is associated with a lower risk of developing PD, as well as can slow down the progression of its physical manifestations (Romenets et al., 2015). Regular exercise has been shown to improve gait speed, strength, functional capacity, and has been shown to reduce falls in PD patients (Romenets et al., 2015). However, many exercise interventions are unappealing for patients with PD and have low compliance and participation (Heiberger et al., 2011a). Since less than half of PD patients meet recommended daily levels of physical activity, there is a need to find interventions involving physical activity that can help patients overcome barriers to participating (Ellis et al., 2013).
Dance in particular, is a promising intervention for PD patients as it offers auditory, visual and sensory stimulation, social interaction, motor learning and memory (Kattenstroth et al., 2010). Compared to other forms of exercise therapy, patients with PD have higher compliance rates when it comes to dancing therapy and is more motivated to attend the classes even after the study period (Hackney and Earhart, 2009). This is particularly important since research shows that exercise interventions are only beneficial if performed regularly over a longer period (Goodwin et al., 2008).
One study by Westheimer et al. from 2015 employed both quantitative measures pre and post-intervention to examine the dance intervention’s effects on motor function and quality of life in Parkinson’s patients. Over eight weeks they found improvements not only in gait and tremor, but the qualitative interviews that took place post-intervention revealed benefits related to the general quality of life and well-being, including improvements in social interactions and increased happiness. However, these results are not consistent across studies. Studies by Hackney and Earhart (2009a), Duncan and Earhart (2012), and Foster et al. (2013) found no differences in gait between participants who had undergone dance intervention and those had no intervention. However, a study by Brichetto et al. (2006) found that scores of Freezing of Gait Questionnaire, but not Unified Parkinson’s Disease Rating Scale (UPDRS) scores significantly improved after treatment, suggesting that the type of quantitative measure being employed in these studies also affects which motor symptoms show improvement.
Limitations
Although there are studies that demonstrate the potential of dance therapy for treating Parkinson’s disorder, there are many discrepancies across studies that make it difficult to know what aspect of the treatment was responsible for improving certain symptoms. For example, many of the studies administered interventions across different timelines. The study by Westheimer et al. ran 16 dance sessions over 8 weeks, while another study by Duncan and Earhart from 2012 ran their study over a course of a year. The frequency of having dance sessions, as well as the duration of each one and the length of the entire study all would influence the impact of the intervention. Looking at the literature, it appears that studies that had longer interventions showed more significant improvements in symptoms compared to the current study. However, there is a lack of understanding of whether longer intervention periods or longer sessions lead to more superior results (de Dreu et al., 2012).
Another important factor that likely influenced the results across studies is whether participants were tested while on medication. Although testing participants while on their best ON medication state can help provide insight into how they perform daily activities, some of the deficits of PD are not fully seen. As a result, these studies lack a full picture of the effects of dance intervention on symptoms, and whether the intervention is disease-modifying (Duncan and Earhart, 2012). Although testing participants while they are on medication appears to be common, as is shown in the meta-analysis by Kathryn Sharp and Jonathan Hewitt (2014), for future studies, testing off medication is warranted. A study by Duncan and Earhart (2012) found improvements in UPDRS scores at 3, 6, and 12 months in patients who were off medication. This allowed them to identify that participation in the dance program had a disease-modifying effect.
Although most dance training employs visual focus, rhythm, and reproducing distinct parts of dance sequences, it is possible that different types and regimes of dance would have different effects on motor symptoms. One study by Westheimer et al. from 2015 employed ballet steps such as tendus, port de bras, and plie in addition to modern, jazz, and tap moves. This is different from many other dance studies that employ tango dancing as an intervention for Parkinson’s patients (Romenets et al, 2015, Hackney et al., 2007a, Hackney et al, 2007b, Hackney and Earhart 2009, Duncan and Earhart, 2012). It would be interesting for future studies to compare different forms of dance on Parkinson’s patients to determine which forms of dance are the most effective and if some forms improve motor symptoms more than others. It is likely that some forms of dance are more effective at treating specific motor symptoms, which has important applications when using dance therapy as treatment. Depending on what symptoms a patient is experiencing, and which are the most severe, a different kind of dance may be prescribed.
Since the study by Westheimer et al. (2015) found improvements in motor symptoms and quality of life aspects reflected in the qualitative interviews but not the quantitative interview, likely, the type of tests used also influence whether improvements are seen. Further research in the field could determine standardized ways to test for the effects of dance interventions on Parkinson’s patients that include both quantitative and qualitative measures.
References for section on dance:
American Music Therapy Association. (2018a). History of music therapy. Retrieved from https://www.musictherapy.org/about/history/
Brancatisano, O., Baird, A., & Thompson, W. (2020). Why is music therapeutic for neurological disorders? The Therapeutic Music Capacities Model. Neuroscience & Biobehavioral Reviews, 112, 600-615. https://doi.org/10.1016/j.neubiorev.2020.02.008
Galvan, A. (2018). Emergent Brain Dynamics: Pre-birth to Adolescence (pp. 225-241). MIT Press.
Hernandez-Ruiz, E. (2019). How is music processed? Tentative answers from cognitive neuroscience. Nordic Journal Of Music Therapy, 28(4), 315-332. https://doi.org/10.1080/08098131.2019.1587785
Hennessy, S., Sachs, M., Ilari, B., & Habibi, A. (2019). Effects of Music Training on Inhibitory Control and Associated Neural Networks in School-Aged Children: A Longitudinal Study. Frontiers In Neuroscience, 13. https://doi.org/10.3389/fnins.2019.01080
Ingersoll, E., & Thoman, E. (1994). The breathing bear: Effects on respiration in premature infants. Physiology & Behavior, 56(5), 855-859. https://doi.org/10.1016/0031-9384(94)90315-8
Loewy, J., & Jaschke, A. (2020). Mechanisms of Timing, Timbre, Repertoire, and Entrainment in Neuroplasticity: Mutual Interplay in Neonatal Development. Frontiers In Integrative Neuroscience, 14. https://doi.org/10.3389/fnint.2020.00008
Peretz, I., & Zatorre, R. (2005). Brain Organization for Music Processing. Annual Review Of Psychology, 56(1), 89-114. https://doi.org/10.1146/annurev.psych.56.091103.070225
Omar, R., Hailstone, J., Warren, J., Crutch, S., & Warren, J. (2010). The cognitive organization of music knowledge: a clinical analysis. Brain, 133(4), 1200-1213. https://doi.org/10.1093/brain/awp345
Siponkoski, S., Martínez-Molina, N., Kuusela, L., Laitinen, S., Holma, M., & Ahlfors, M. et al. (2020). Music Therapy Enhances Executive Functions and Prefrontal Structural Neuroplasticity after Traumatic Brain Injury: Evidence from a Randomized Controlled Trial. Journal Of Neurotrauma, 37(4), 618-634. https://doi.org/10.1089/neu.2019.6413
Tai, S., & Kuo, Y. (2019). Alterations in brainwaves caused by different Music Genres, 1-4. https://doi.org/10.1109/ICAwST.2019.8923257
Trimble, M., & Hesdorffer, D. (2017). Music and the brain: the neuroscience of music and musical appreciation. BJPsych international, 14(2), 28–31. https://doi.org/10.1192/s2056474000001720
Vik, B., Skeie, G., & Specht, K. (2019). Neuroplastic Effects in Patients With Traumatic Brain Injury After Music-Supported Therapy. Frontiers In Human Neuroscience, 13. https://doi.org/10.3389/fnhum.2019.00177
Vik, B., Skeie, G., Vikane, E., & Specht, K. (2018). Effects of music production on cortical plasticity within cognitive rehabilitation of patients with mild traumatic brain injury. Brain Injury, 32(5), 634-643. https://doi.org/10.1080/02699052.2018.1431842
Walton, K. (1988). What Is Abstract about the Art of Music?. The Journal Of Aesthetics And Art Criticism, 46(3), 351-364. https://doi.org/10.2307/431106
References for section on music:
Burzynska, A.Z., Finc, K., Taylor, B.K., Knecht, A.M., Kramer, A.F. (2017). The Dancing Brain: Structural and Functional Signatures of Expert Dance Training. Frontiers in Human Neuroscience 11(56): 1-20.
Brichetto, G., Pelosin, E., Marchese, R., Abbruzzese, G. (2006). Evaluation of physical therapy in parkinsonian patients with freezing of gait: a pilot study. Clin Rehabil, 20, 31-35.
De Dreu, M.J., Vdw, A.S.D., Poppe, E., Kwakkel, G., van Wegen, E.E.H. (2012). Rehabilitation, exercise therapy and music in patients with Parkinson’s disease: a meta-analysis on the effects of music-based movement therapy on walking ability balance and quality of life. Parkinson Relat Disord 19(1), S114-S9.
Duncan, R.P., Earhart, G.M. (2012). Randomized Controlled Trial of Community-Based Dancing to Modify Disease Progression in Parkinson Disease. Neurorehabilitation and Neural Repair, 26(2),132-143.
Ellis, T., Boudreau, J.K., Deangelis, T.R., Brown, L.E., Cavanaugh, J.T., Earhart G.M., et al. (2013). Barriers to exercise in people with Parkinson disease. Phys Ther, 93, 628-636.
Foster, E., Golden, L., Ryan, P., Duncan, R., Earhart, M. (2013). A community-based argentine Tango dance program is associated with increased activity participation among individuals with Parkinson disease. Arch Phys Med Rehab, 94(2), 240-249.
Goodwin V., Richards S., Taylor R., Taylor A., Campbell J. (2008). The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis, Mov Disord 23(5), 631-640.
Hackney, M.E., Earhart, G.M. (2009). Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med, 41, 475-81.
Hackney, M.E., Earhart, G.M. (2009a). Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat Disord, 15, 644–648.
Heiberger, L., Cristoph, M., Amtage, F., Mendez, Balbuena, I., Schulte-Monting, J., Heppo-Retmond, M.C., Kristeva, R. (2011a). Impact of a weekly dance class on the functional mobility and on the quality of life in individuals with Parkinson’s disease. Front Aging Neurosci, 3(14), 1-15.
Hui, E., Chui, B., Woo, J. (2009). Effects of dance on physical and psychological well being in older persons. Arch Gerontol Geriatr, 49(1), e45-d50.
Kattenstroth, J.C., Kolankowska, L., Kailish, T., Dinse, H. (2010). Superior sensory, motor, and cognitive performance in elderly individuals with multi-year dancing activities. Front Aging Neurosci, 2(31), 1-9.
Kulinna, P.H., Stylianou, M., Dyson, B., Banville, D., Dryden, C., Colby, R. (2018). The Effect of an Authentic Acute Physical Education Session of Dance on Elementary Students’ Selective Attention. Biomed Research International 2018: 1-8.
Nemes, B.A., Pirlog, R., Tartamus, D., Capusan, C., Fodor, D.M. (2019). The role of dance therapy in the rehabilitation of Parkinson’s disease patients. Balneo Research Journal, 10(3), 300-304.
Rehfeld, K., Luders, A., Hokelmann, A., Lessman, V., Kaufmann, J., Brigadski, T., Muller, P., Muller, N.G. (2018). Dance training is superior to repetitive physical exercise in inducing brain plasticity in the elderly. PLoS ONE 13(7): 1-15.
Romenets, S.R., Anang, J., Fereshtehnejad, S.M., Pelletier, A., Postuma, R. (2015). Tango for treatment of motor and non-motor manifestations in Parkinson’s disease: A randomized control study. Complementary Therapies in Medicine, 23, 175-184.
Sharp, K., Hewitt., J. (2014). Dance as an intervention for people with Parkinson’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev 47: 445-56.
Teixeira-Machado, L., Arida, R.M., de Jesus Mari, J. (2019). Dance for neuroplasticity: A descriptive systematic review. Neuroscience and Behavioral Reviews 96: 232-240.
Westheimer O., McRae C., Henchcliffe C., Fesharaki A., Glazman S., Ene H., Bodis-Wollner I. (2015). Dance for PD: a preliminary investigation of effects on motor function and quality of life among persons with Parkinson’s Disease (PD). J Neural Transm, 122, 1263-1270.


