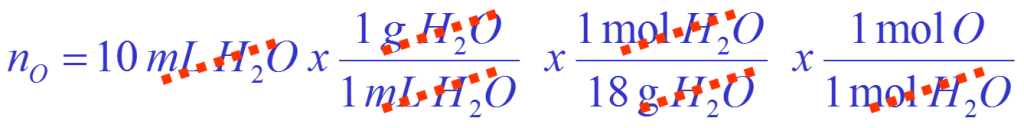3 Chemical Problem Solving Strategies
Unit Analysis and Problem Solving
A ‘book-keeping’ method for units in a calculation |
Over all method of “unit analysis”: |
| Unit analysis helps avoid errors in a multi-step calculation and provides the units for the final answer. |
1) Write the units with every number you include in a series of calculations 2) String your calculations together as a series of multiplications or divisions before doing any math 3) Cancel your units to see the calculation evolve |
Calculations: Converting from One Unit to Another |
|
Unit analysis: |
Conversion factor: |
|
A method that uses a conversion factor to convert a quantity expressed in one unit to an equivalent quantity in a different unit. |
States the relationship between two different units. |
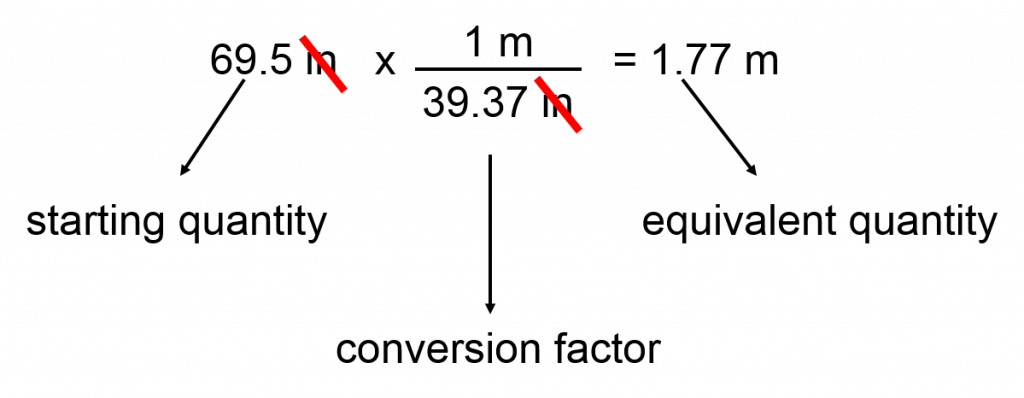
original quantity x conversion factor = equivalent quantity
For example converting between length units
Given that 1 meter = 39.37 inches
Conversion factors 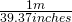 or
or 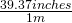
The same relationship, just invert as necessary to give you the units you need!
Calculations: Using Unit Analysis
FACT:
The more you use the “long method” of converting units, the fewer errors you will make!
Problem Solving Examples
How many moles of oxygen atoms are there in a 10 mL volume of water?
What is being asked? |
What data is provided? |
What do I need to know? |
How do I need to state the answer? |
| Given a volume can you calculate a number of atoms? | Data: 10 mL of water | Need to know: water is |
Answer in moles of oxygen O |
Convert volume of water to moles of oxygen
Calculation is: Volume of ![]() mass of
mass of ![]() mols of
mols of ![]() mols of
mols of ![]()
= There are 0.55 moles of oxygen atoms.
Always Check Units!
Examples
Problems Set
Below are two documents. One is practice problems, the second is the same problems with solutions.
They can be downloaded and changed to suit your needs.
| Problem Set | Problem Set Solutions |
Quiz