1 Scientific Measurements
Accurate measurements are essential when performing scientific experiments, this unit discusses accepted methods of measurement used by scientists, and the importance of units.
Scientific measurements generally adhere to the International System of Units (SI units). It is important to always include units when recording data, doing calculations and reporting results! Units are globally recognized and necessary for sharing information between scientists around the world. If you are unfamiliar with units of measurement, take time now to go through this interactive table to learn the definitions and meaning of the SI units that are commonly used in chemistry .
Scientific Measurements
Accuracy: |
Precision: |
|
How close a measured value is to the accepted or real value. |
The degree of reproducibility of a measured quantity; how close a series of measurements of the same quantity are to one other. |
Scientific measurements generally adhere to the International System of Units (SI).
SI Base Units
Click on the following units of measurement to reveal their definition.
Note: Precise definitions of the SI units are not necessary to memorize. Rather, the relationships between the units and how to use them are the important parts to know.
SI Derived Units
Click on the following units of measurement to reveal their definition.
Volume
Volume is a measure of space. It is a unit of length raised to the third power.
The SI unit of length is the meter. One meter cubed is equivalent to 1000 L. Litres, which are a convenient unit for scientific measurements, are a more common measurement unit than meters cubed.
 |
 |
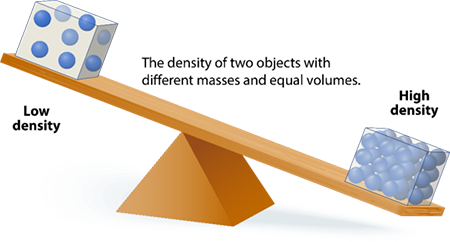
Density
A ratio of mass (m) to volume (V) of a substance.
![]()
SI Prefixes: Units of Measure for All Sizes
The dimensions of the different types of matter measured in scientific research vary a lot! It would be impractical to use a macroscopic measurement, such as the meter, to report the length of a human cell. To keep measurements in a usable scale, scientists use SI prefixes, which are multipliers that change unit values by multiples of ten. For example, a nanometer is nine orders of magnitude smaller than a meter and is convenient for measurements of objects such as molecules.
Multipliers that change unit values by multiples of ten.
![]()
![]() = 1000000000000 nm
= 1000000000000 nm
Scientific Measurements Examples
When making scientific measurements it is important to be as precise as possible. Precision is the degree of reproducibility of a measured quantity; how close a series of measurements of the same quantity are to one other. A measurement value with a high degree of precision will have a larger number of digits.
Scientific Measurements Problem Set
Below are two documents. One is practice problems, the second is the same problems with solutions.
They can be downloaded and changed to suit your needs.
| Problem Set 1 | Problem Set 1 Solutions |
Scientific Measurements Quiz