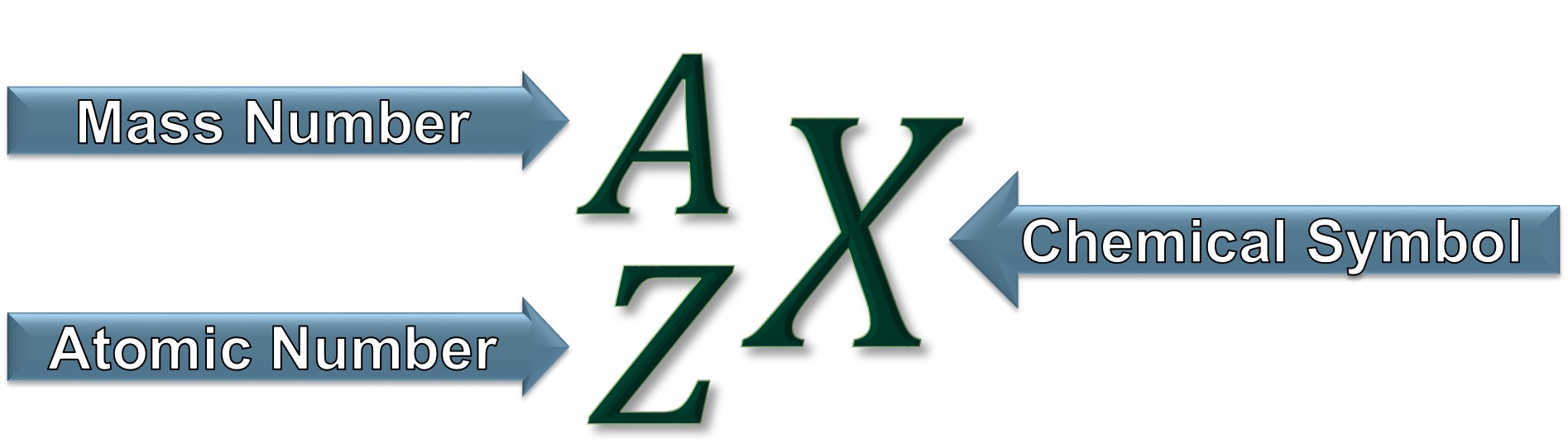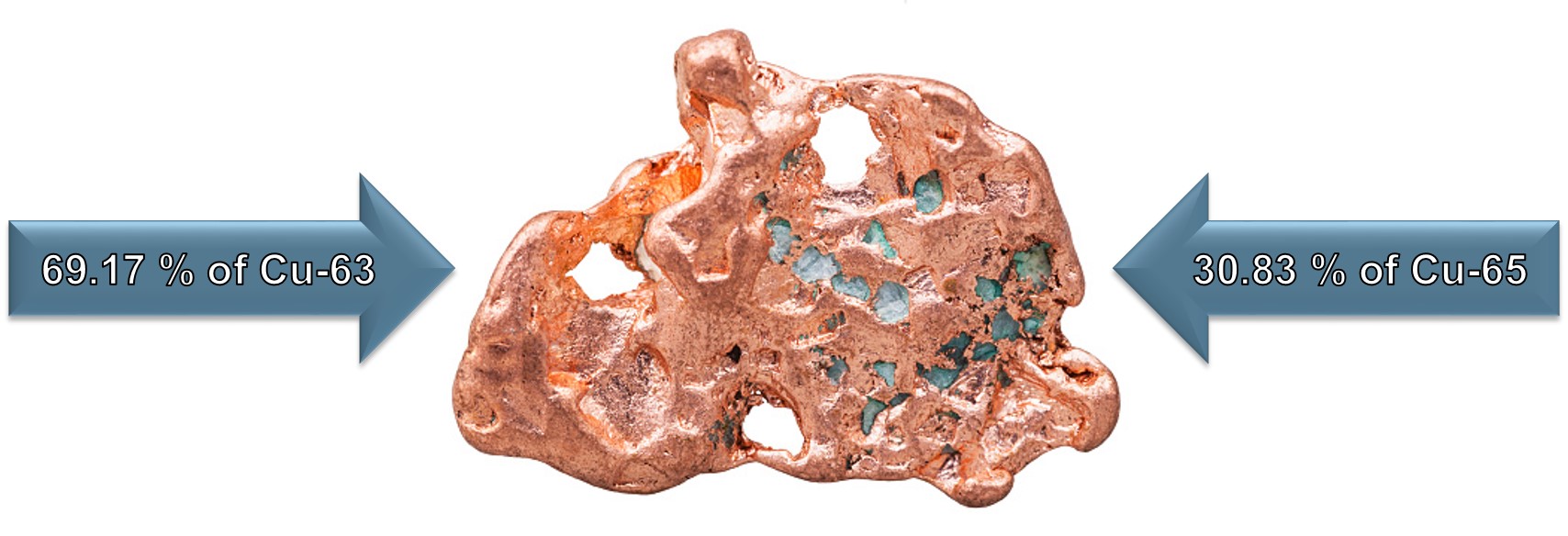5 Modern Atomic Theory
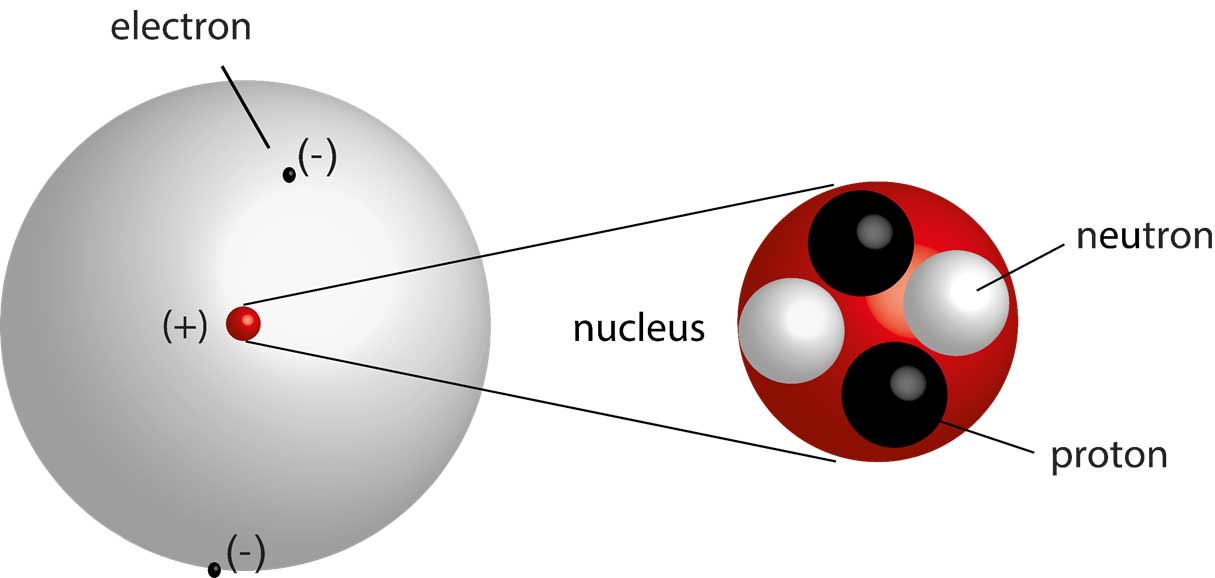
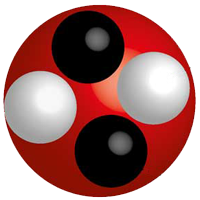
The Atom
The Atom
A neutral atom has the same number of protons as electrons.
Physical Properties of Sub-atomic Particles
| Particle | Charge (Coulombs) | Mass (kilograms) | Relative Charge | Mass (unified atomic mass units) |
| Proton | ||||
| Neutron | No Charge | |||
| Electron |
Unified atomic mass units (u):
| Using this measurement, the mass of a proton and neutron is approximately |
The masses of all elements are defined using unified atomic mass units. |
![]() the mass of a carbon atom.
the mass of a carbon atom.
Atom Terminology
Z = number of protons = atomic number
A = number of protons + number of neutrons = mass number
Element |
Chemical Symbol (X) |
Atomic Number (Z) |
Mass Number (A) |
| One or more atoms of the same type. | A one or two letter abbreviation assigned to each element. | The number of protons in the nucleus of an atom. The type of atom/element is determined by the number of protons. | The sum of the number of neutrons and protons in the nucleus of an atom. |
The number of protons defines the type of atom or element!
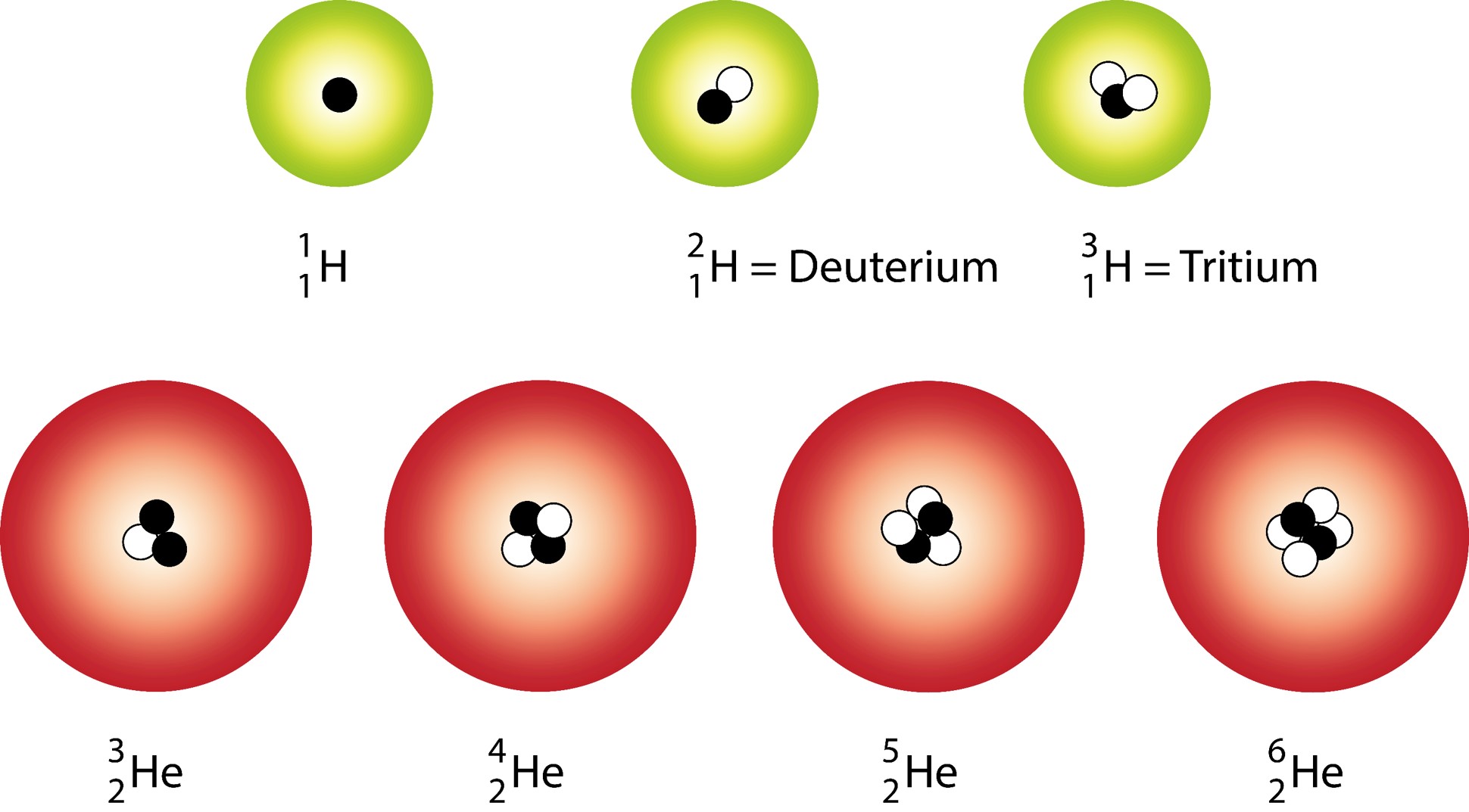
Isotopes
Isotopes are atoms of the same type that have the same number of protons and varying numbers of neutrons.
We know: |
Isotopes: |
|
The number of protons determines the type of atom or element. Neutrons and protons comprise the nucleus of an atom and have the same approximate mass. |
The number of protons determines the type of atom or element. Isotopes of elements are distinguished by the mass number (A). |
Isotopes Example:
Atoms of an element that have a different number of neutrons.
 |
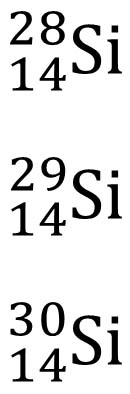 |
28-14=14 neutrons29-14=15 neutrons30-14=16 neutrons |
Isotope Notation
Including the atomic number is redundant when representing isotopes. It is common to use the following notation: X-A.
For example: Si-28, Si-29, Si-30
Atomic Mass
We know: |
Atomic Mass: |
|
Isotopes are atoms of the same type that have a different number of neutrons. The mass of an atom is dictated by the mass of protons and neutrons because the mass of an electron is negligible in comparison. |
Is the weighted average of isotopic masses based on the natural abundance of each isotope. The natural abundance is the relative amount of each isotope found in a natural sample of any element. Natural abundance is relatively constant and unique for each element. |
The atomic mass of an element as seen on the periodic table is a weighted average of isotopic masses of that element.
Atomic Mass Example:
This Copper (Cu) sample will contain approximately 30.83 % of Cu-65 and 69.17 % of Cu-63
Atomic Mass Calculations:
Atomic mass for an element can be calculated with the following formula:
![]()
In simple terms this formula is telling us that atomic mass can be calculated by:
|
1) Multiplying each isotopic mass of an element by its relative abundance. |
2) Adding the resulting masses of the isotopes together. |
An atomic mass calculation is included in the example section!
The Mole
A measurement similar to a pair, dozen or score. A pair is 2 objects, a dozen is 12 objects and a score is 20 objects.
![]() objects = Avogadro’s number
objects = Avogadro’s number
Defining the Mole:
The mole is defined using the C-12 isotope.
One mole = number of atoms in exactly 12 g C-12.
12 g of C-12 = 1 mole of C-12 atoms = exactly ![]() C-12 atoms
C-12 atoms
The mass of one mole of C-12 atoms is 12 g
1 mole of carbon (C) = ![]() atoms
atoms
Molar Mass:
Using C-12 as a standard for the mole gives a relationship between mass, number of atoms, and unified atomic mass units.
The mole can be used to count atoms based on their mass.
The molar mass of an element is the mass of one mole of each element.
 |
 |
 |
| 1 mole of carbon (C) |
1 mole of sulfur (S) |
1 mole of gold (Au) |
Molar Mass of an Element:
The molar mass of an element is the mass of one mole of each element.
Molar mass the mass of the x atoms of each element.
Molar mass is numerically equivalent to an element’s mass in unified atomic mass units .
Molar Mass ![]() is numerically equivalent to atomic mass
is numerically equivalent to atomic mass ![]()
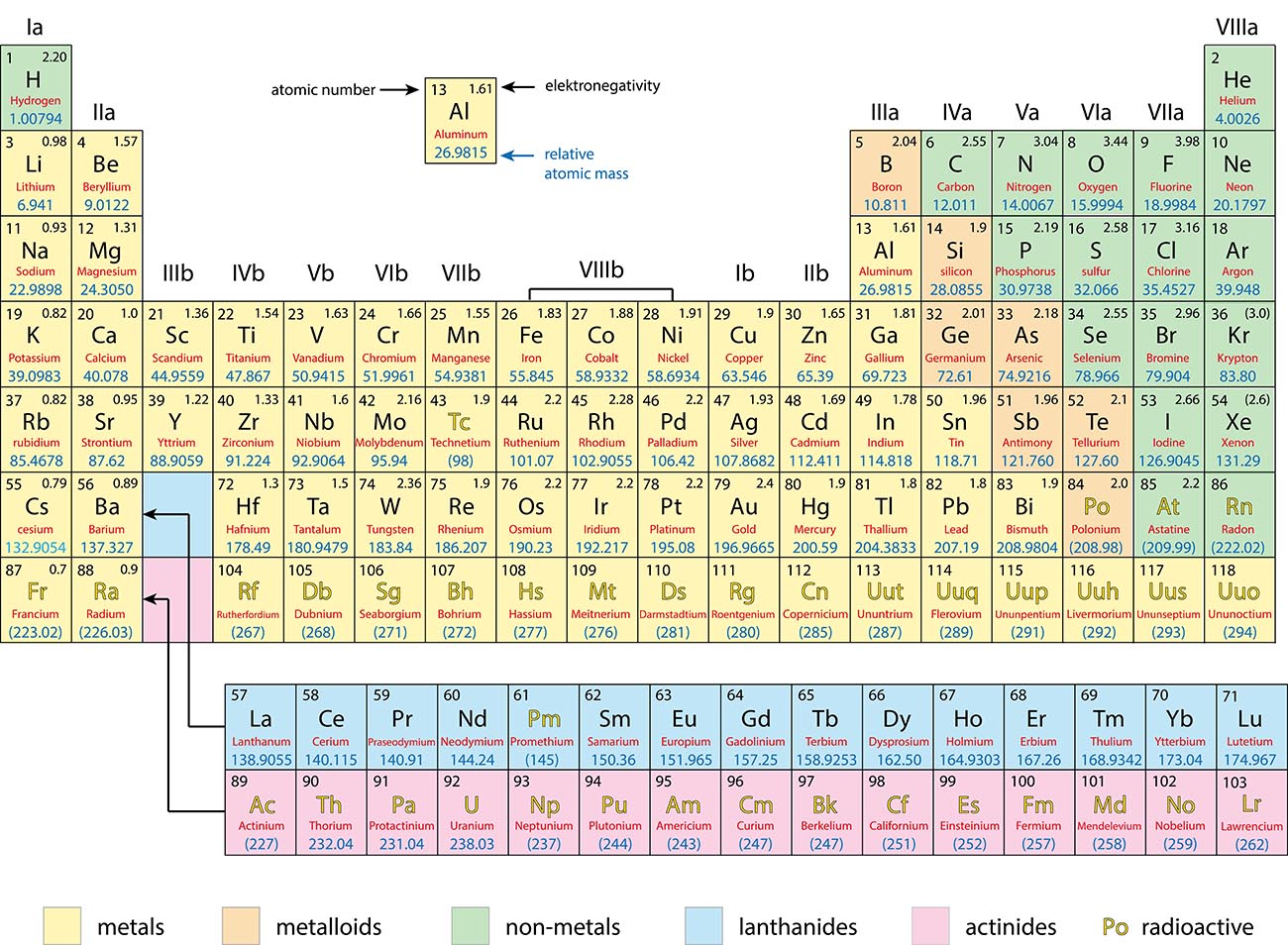
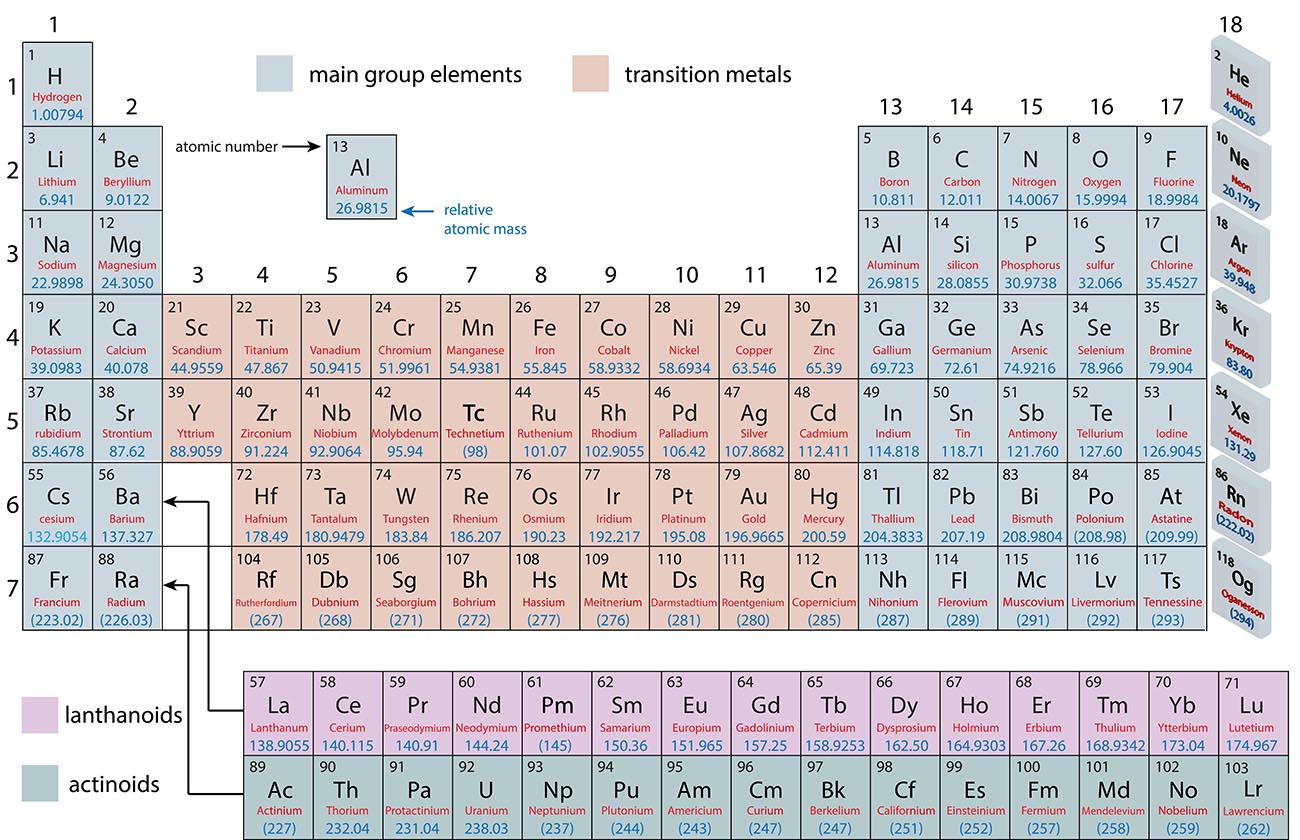
The Periodic Table of Elements
Metals, Non-Metals and Metalloids
Periodic Trends
Metals: |
Non-Metals: |
Metalloids: |
|
Generally good conductors of heat and electricity Tend to lose electrons in a chemical reaction |
Generally poor conductors of heat and electricity Tend to gain electrons in a chemical reaction |
Have properties of both metals and non-metals. |
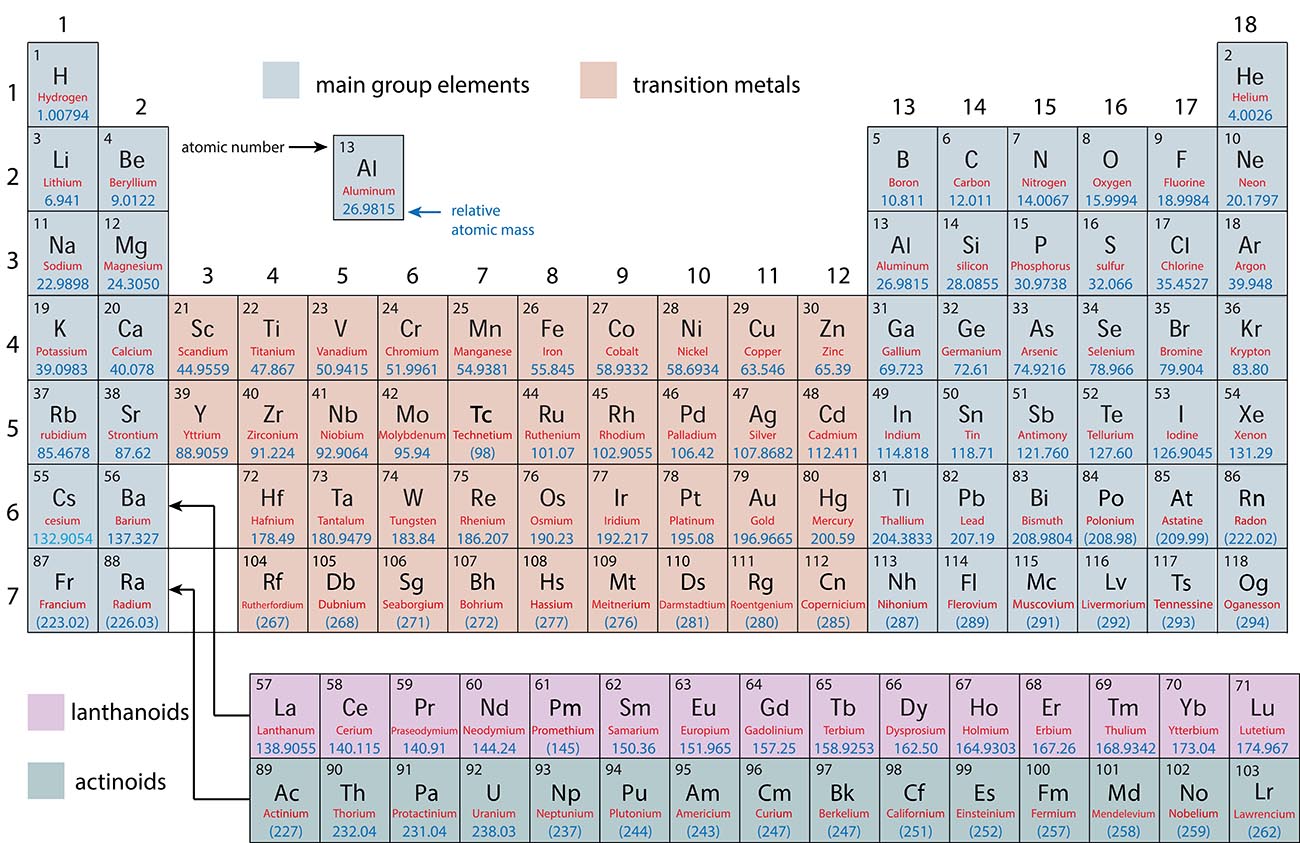
Main Group Elements and Transition Metals
Periodic Trends
Main Group: |
Transition Metals: |
Lanthanoids and Actinoids: |
|
Elements that have predictable properties |
Elements that have unpredictable properties. |
Elements that are placed separately for a more compact periodic table. They have similar properties to the elements lanthanum and actinium. |
Groups and Periods of the Periodic Table
Periodic Trends
|
Each column in the periodic table is called a group and numbered 1-18 |
Each row in the periodic table is called a row and numbered 1-7 |
The elements in a group have similar properties. |
For example: The noble gases in group 18 are all relatively unreactive. |
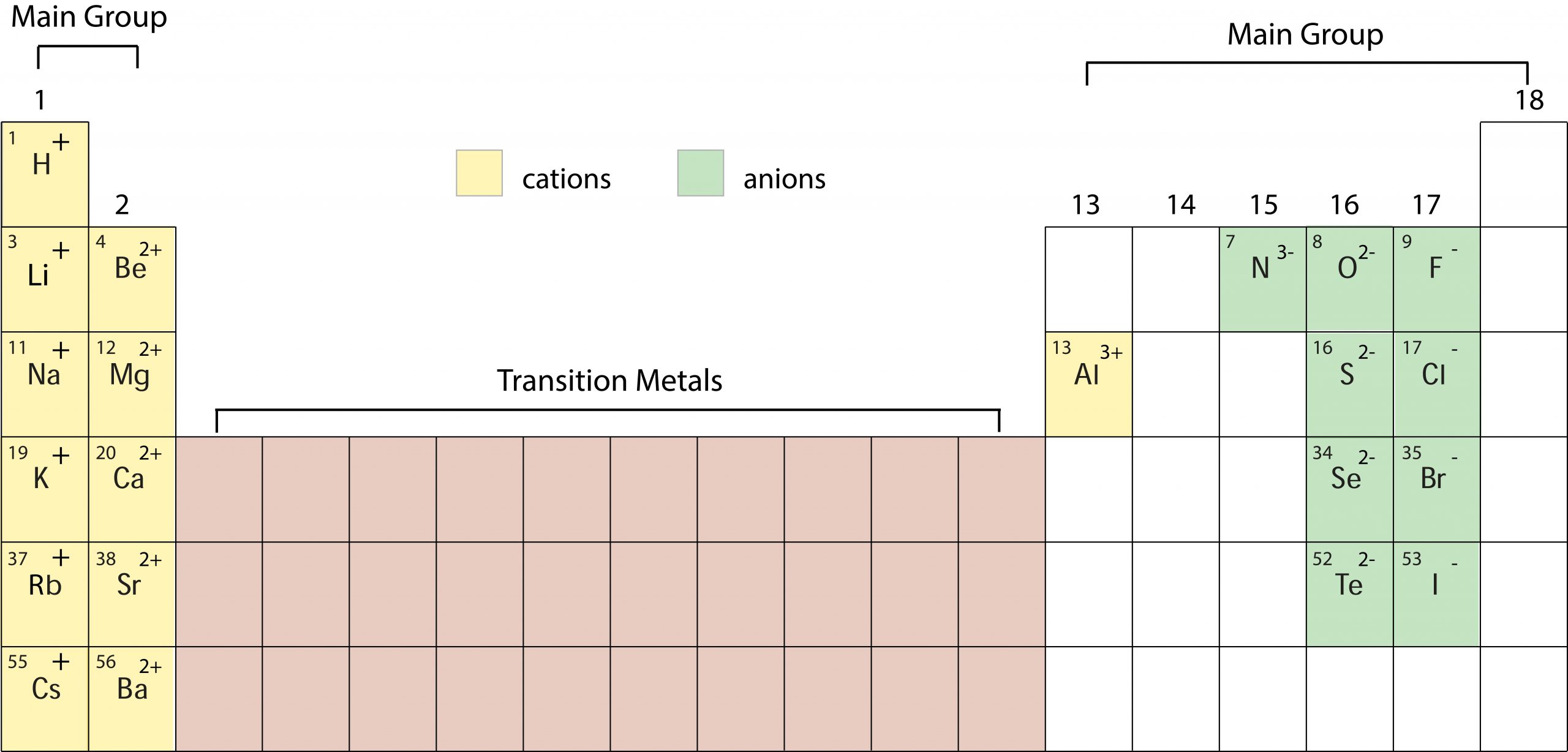
Ions
Common Main Group Ions:
We know: |
Ions: |
Ions and Groups: |
|
The number of protons determines the type of atom or element. Neutrons and protons comprise the nucleus of an atom, and have the same approximate mass. |
One or more atoms that has gained or lost an electron(s). Ions are charged particles. The periodic table can help predict which ions will form. |
A main group non-metal will generally gain electrons. A main group metal will generally lose electrons. |
Ions are charged particles that have gained or lost an electron(s).
Cation (+): |
Anion (-): |
Ion Notation: |
|
One or more atoms that has lost an electron(s). A main group METAL will LOSE electrons to form a CATION with the same number of electrons as the nearest noble gas. |
One or more atoms that has gained an electron(s). A main group NONMETAL will GAIN electrons to form an ANION with the same number of electrons as the nearest noble gas. |
Ions are represented as the chemical symbol of the ion with the charge of the ion noted as a superscript to the right.
Lithium Ion = |
Examples
The following examples will walk you through solving a problem.
Example Problem 1
Calculate the average atomic mass of silicon given the information in the table.
| Isotope | Percent Natural Abundance (%) | Mass (u) |
| Si-28 | 92.2 | 27.9769 |
| Si-29 | 4.7 | 28.9764 |
| Si-30 | 3.1 | 29.9738 |
Example Problem 2
These gold earrings have a mass of 1.00 g. Calculate the moles of gold in the earrings, how many gold atoms do the earrings contain? Assume the earrings are pure gold. |
 |
Problem Set
Below are two documents. One is practice problems, the second is the same problems with solutions.
They can be downloaded and changed to suit your needs.
| Problem Set | Problem Set Solutions |
Quiz
Atomic Structure
Atom Models
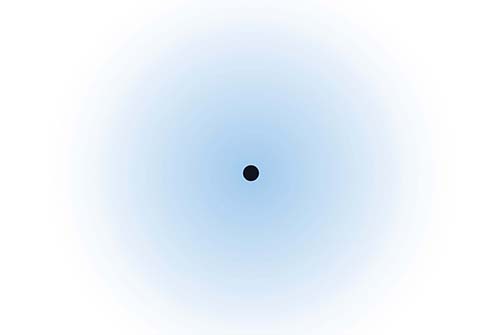
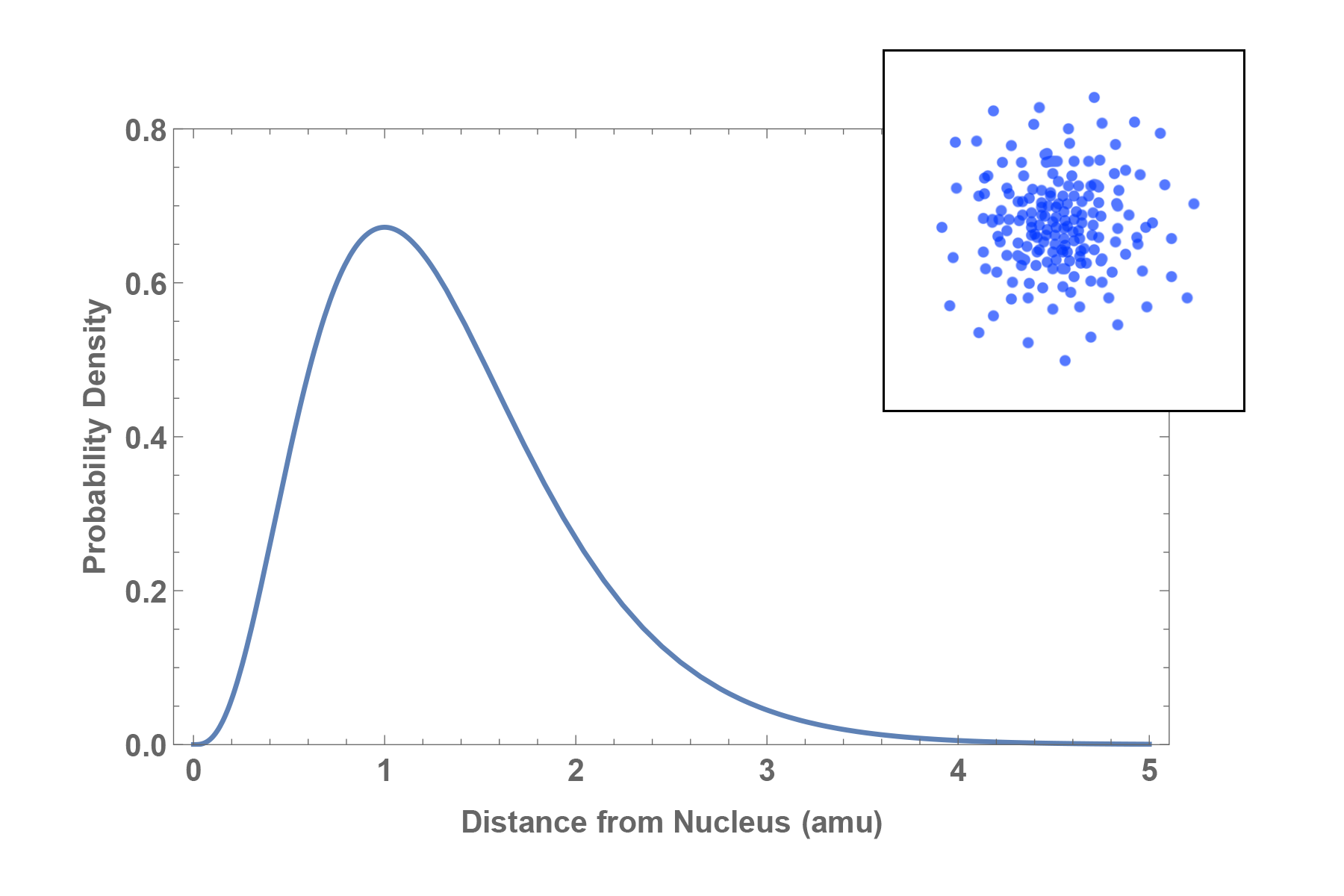
Orbital:
The likely position of an electron in an atom described by a probability distribution map.
Quantum Numbers = Electron’s “Address”
Quantum Numbers
Principal Quantum Number (n) |
Angular Momentum Quantum Number (l) |
Magnetic Quantum Number
|
|
Orbital’s size and energy level of an electron. A whole number 1,2,3,4….. n increases with an electron’s energy and distance from the nucleus. Each level is called a shell. |
The shape of an orbital. 0,1,2,3,…,n-1 Each value for l is a subshell Each number value of l is assigned a letter s,p,d,f,g…. |
Orientation of orbital in 3D space. Value= integer values from –l…l. There are 2(l)+1 values of |
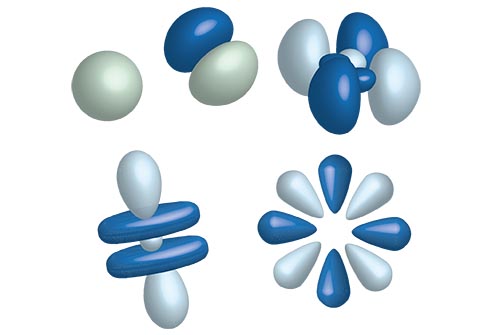
Orbital Shapes
Orbital Shapes = Angular Momentum Number (l)
| Values of (l) angular momentum | |
| Number | Letter |
| 0 | s |
| 1 | p |
| 2 | d |
| 3 | f |
| 4 | g |
Letter values continue according to the alphabet after f, with the exception of J.
s,p,d,f,g,h,i,k,….. Sober Physicists Don’t Find Giraffes Hiding In Kitchens
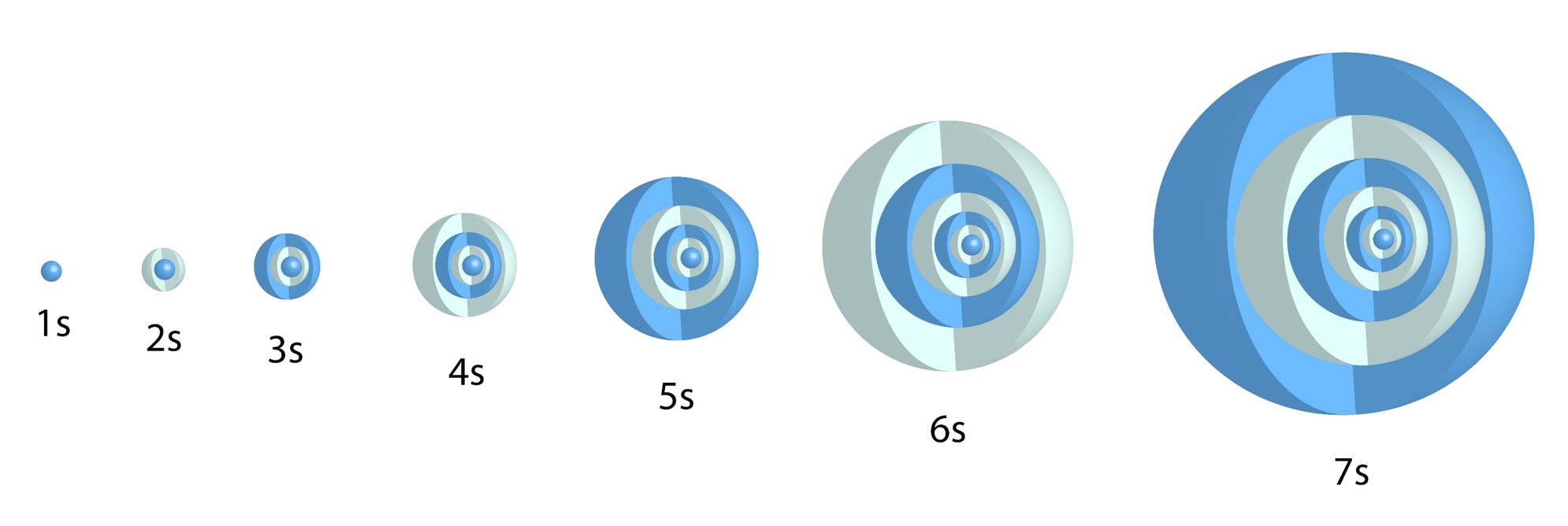
s Orbital:
![]()
One allowed value for ![]() = one type of s orbital for each value of n.
= one type of s orbital for each value of n.
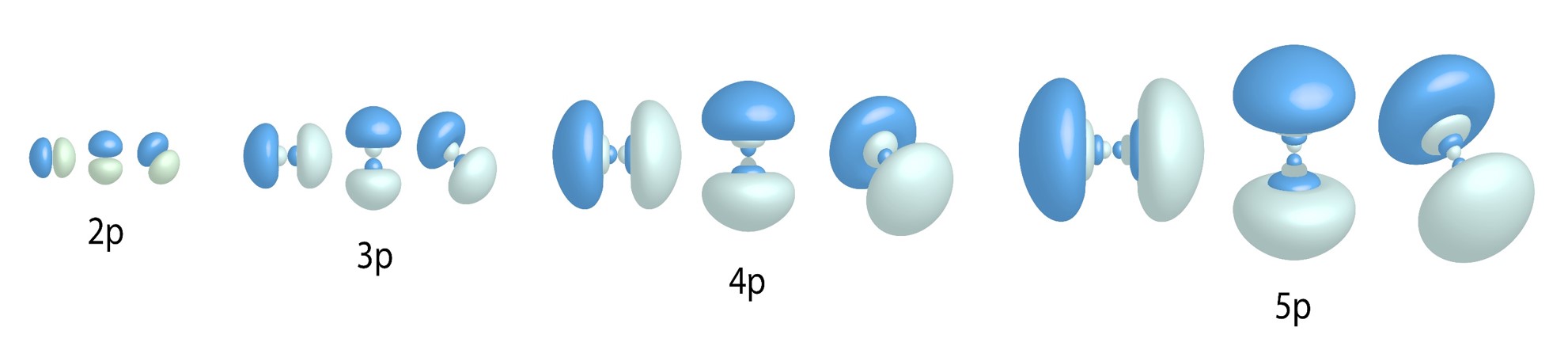
p Orbitals:
![]()
Three allowed values for ![]() = three types of p orbitals for n values greater than and equal to two.
= three types of p orbitals for n values greater than and equal to two.
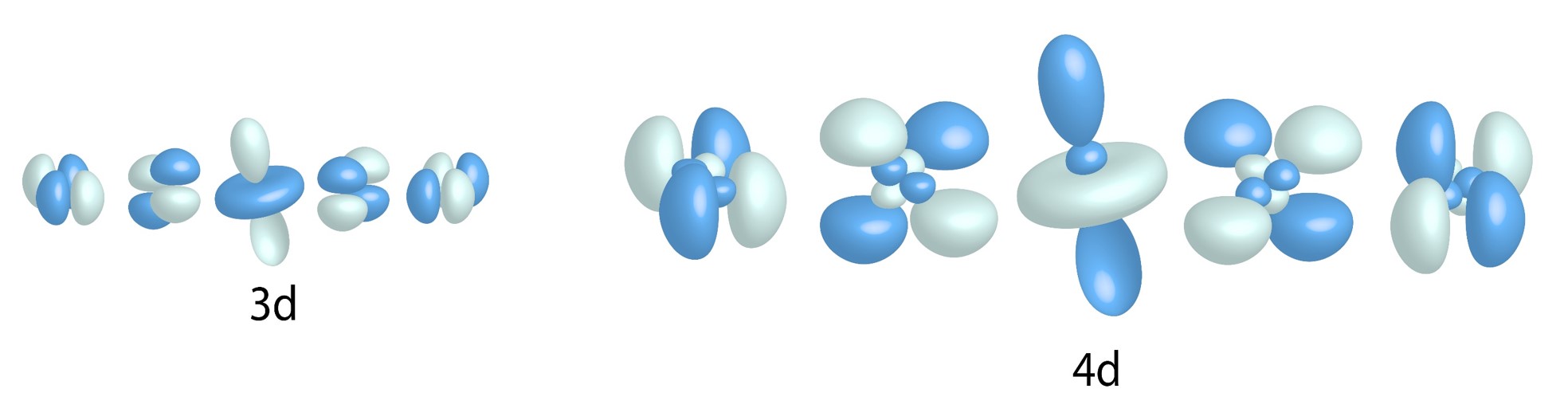
d Orbitals:
![]()
Five allowed values for ![]() = five types of d orbitals for n greater than and equal to three.
= five types of d orbitals for n greater than and equal to three.
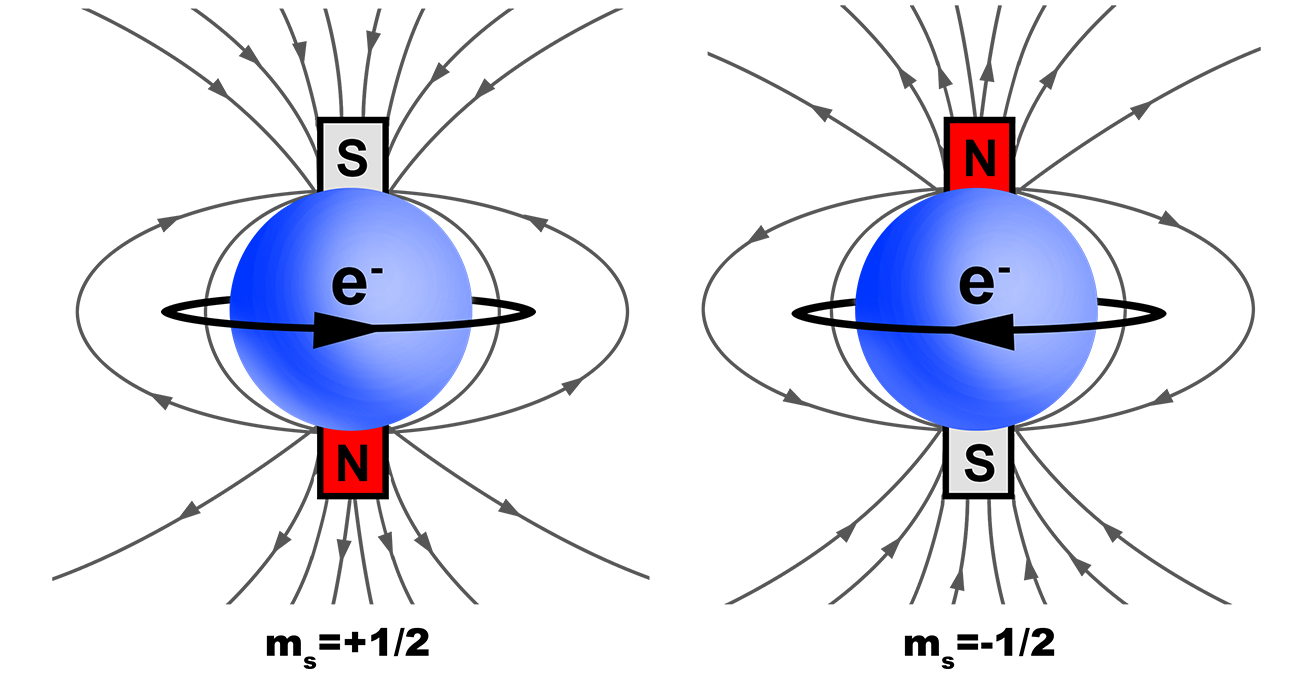
Spin Quantum Number 
Spin is intrinsic to all electrons, it is quantized, with only two orientations; spin up or spin down.
Value = ![]() OR
OR ![]()
Pauli Exclusion Principle
No two electrons in an atom can have the same four quantum numbers, however, Any three quantum numbers can be the same.
This implies: One orbital may contain two electrons of opposite spin orientation!
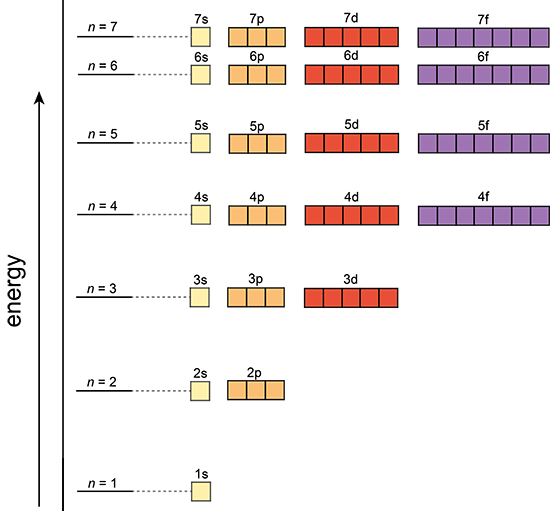
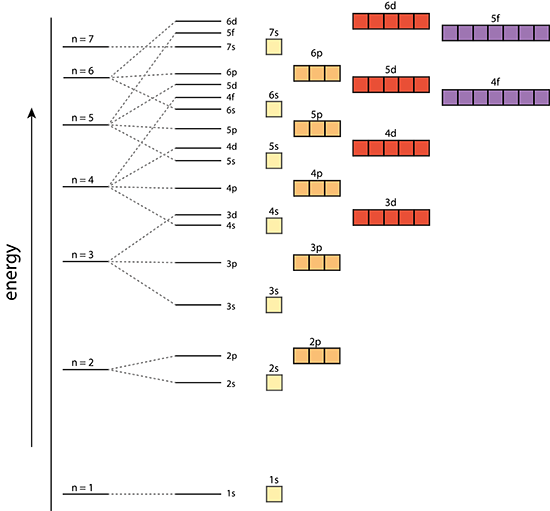
Aufbau Principle
In a one electron system the subshells are degenerate; meaning s,p,d,f,.., all have the same energy level in each shell. However, in a multi-electron atom the subshells have different energy levels.
 |
 |
Single electron atom |
Multi-electron atom |
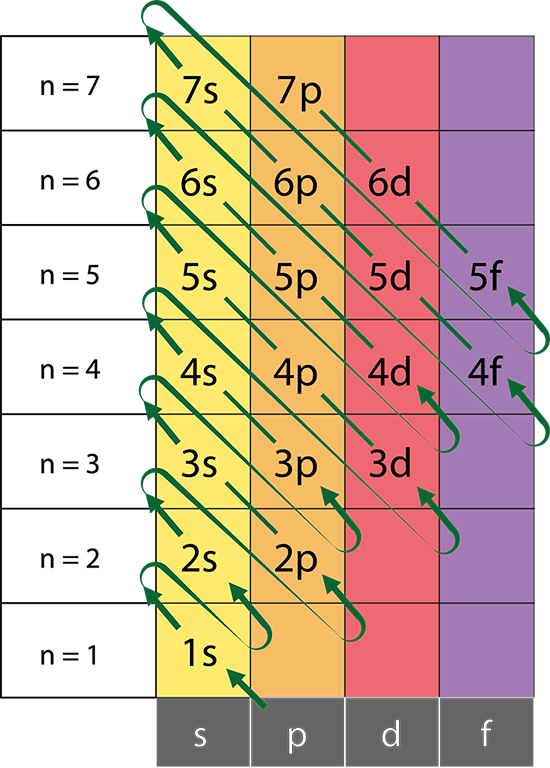
The Aufbau Principle:
That means that we want to know about a system in which the lower energy orbitals fill before the higher energy orbitals. This idea is referred to as the Aufbau principle, and the general order in which electrons fill orbitals in an atom to achieve the ground state configuration is: 1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p
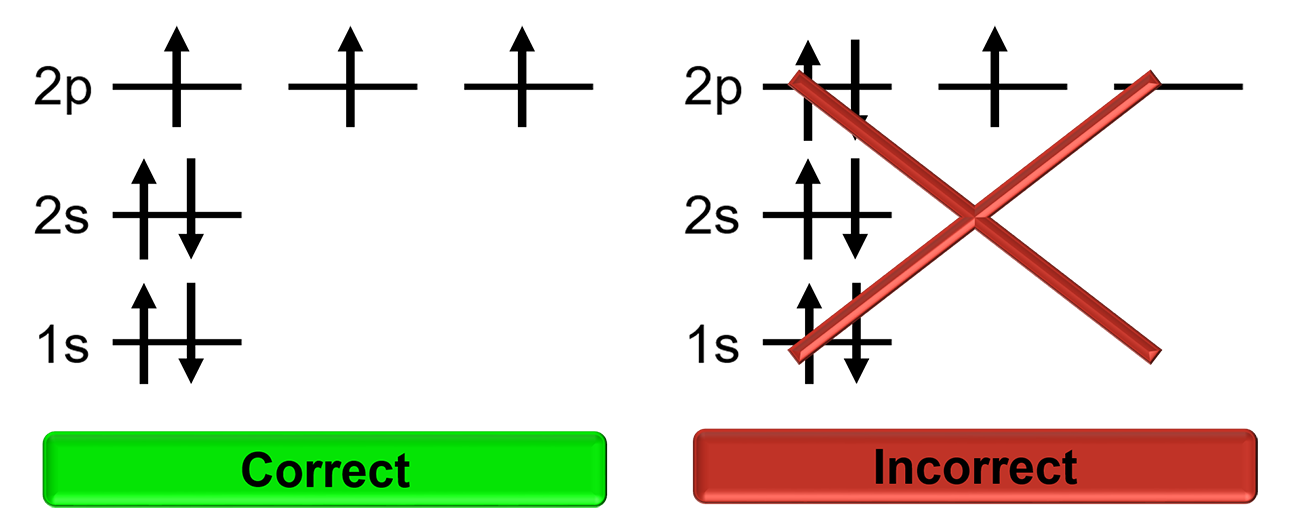
Hund’s Rule
Electrons will singly occupy degenerate orbitals until they are half full before pairing up with other electrons. Electrons in half full orbitals will have the same spin.
Electronic Configurations
A notation that represents the orbitals occupied by electrons in an atom.
Write the value of n, followed by the number of electrons allowed in each orbital until all electrons in the atom are assigned to an orbital.
Example Carbon: 6 electrons = ![]()
Core Electrons |
Valence Electrons |
Short-hand Electronic Configuration |
| Electrons in a filled inner shell. | Electrons in the outermost shell (highest n value). Example: Carbon 6 electrons = |
An electronic configuration using a noble gas to represent the filled shells and only writing out the valence electrons. |
Orbital Diagrams
A diagram that represents the filled orbitals in an atom by denoting the electron as an arrow.
Each subshell is represented as a line or a circle, electrons are represented as arrows pointing up or down according to the electron’s spin.
| Example Carbon: 6 electrons = |
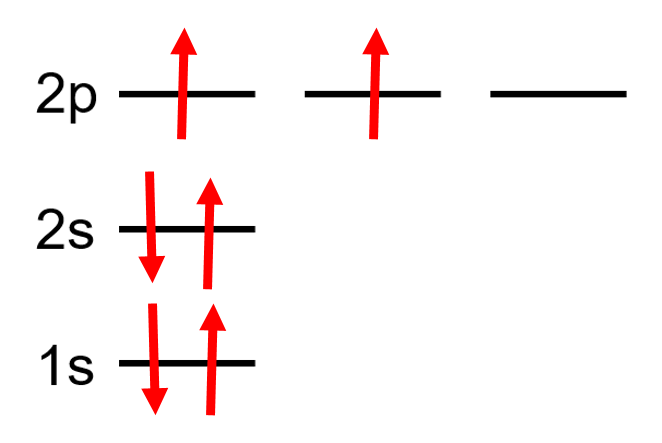 |
Examples
The following video will help you understand electron configurations and orbital diagrams
Problem Set
Below are two documents. One is practice problems, the second is the same problems with solutions.
They can be downloaded and changed to suit your needs.
| Problem Set | Problem Set Solutions |