Vaccine Components
The components of vaccines used in Canada are extremely safe. See Image 1.4 for an illustration of vaccine components and Table 1.2 for a description of the components.
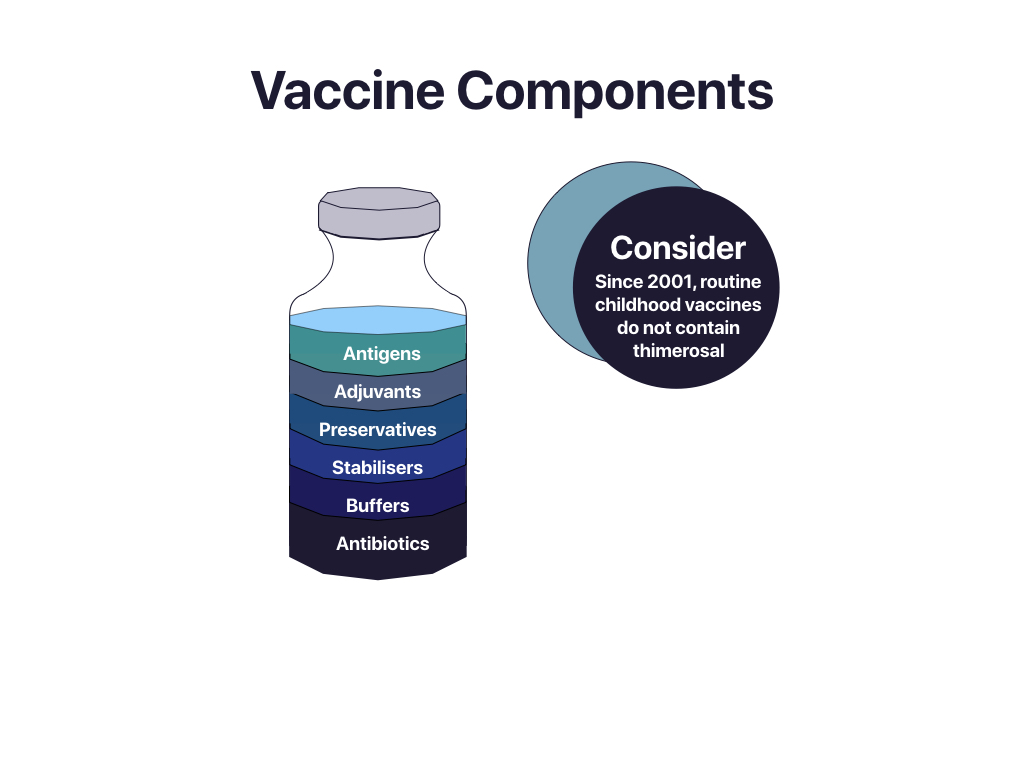
Serious side effects like allergic reactions are very rare. Chemicals used in vaccines enhance their effectiveness, preserve or stabilize the pathogen, kill unwanted viruses, or act as a suspension to hold the product. Some vaccines contain trace amounts of culture material like egg protein used to grow the virus or bacteria. It is important for health professionals to familiarize themselves with some of the common components (also referred to as ingredients) in vaccines, particularly if a client has an allergy or expresses concern in this regard. For a comprehensive list of vaccines and their components visit Canadian Immunization Guide, Key Immunization Information.
Table 1.2: Vaccine Components
| Component | Description |
|
Antigen
|
The active component of the vaccine that causes an immune response.
|
|
Adjuvants
|
Component that enhances the vaccines’ effectiveness. Most vaccines use aluminum-based adjuvants. They induce a range of inflammatory factors to the injection site which helps the immune response. Aluminum is removed from the body in urine via the kidneys.
|
|
Preservatives
|
Preservatives stop unwanted contamination of a vaccine. The most common preservative is 2-phenoxyethanol, which is also used in a range of cosmetics, baby care products, eye and ear drops. Clients may be concerned about the presence of Thimerosal, a mercury-containing preservative used in multi-dose vaccines to prevent contamination of virulent bacteria or fungus. Contrary to popular belief, most vaccines in Canada do not contain Thimerosal. School-required vaccines have not contained Thimerosal since 2001.
|
|
Stabilizers
|
Stabilizers, such as gelatin, stop chemical reactions from occurring in the vaccine and prevent the components from separating. Other stabilizers include amino acids (the building blocks of proteins), potassium, sodium, and lactose.
|
|
Buffers
|
A buffer solution resists changes in pH when small quantities of an acid or an alkali are added to it. Buffers keep the vaccine at a similar pH to the body. Often the buffer will be a salt.
|
|
Adjusting tonicity
|
To keep the vaccine isotonic (to reduce local reactions) a salt may be added. Often this will be Sodium Chloride (common table salt).
|
|
Surfactants and emulsifiers
|
These agents act like a detergent. A commonly used surfactant is called Polysorbate 80 or Tween®. This is made from sorbitol (a sugar alcohol) and Oleic acid (an omega fatty acid). Polysorbate is often used in foods such as ice cream.
|
|
Formaldehyde
|
Formaldehyde is used to detoxify or inactivate the live germ or toxin used in some vaccines. It is mostly removed during the purification process. It is important to note that trace amounts of Formaldehyde is found in all human bodies and is required for the synthesis of DNA. Formaldehyde breaks down very quickly both in the environment and in the body. Additionally, there is about ten times the amount of formaldehyde in a baby’s body than there is in a vaccine.
|
|
Antibiotics
|
Some vaccines contain antibiotics to prevent bacterial growth during storage of the vaccine.
|
Points of Consideration
There is a common misconception about the types of mercury contained in vaccines and the levels of toxicity. As health professionals, it is important to resolve public misconceptions through accurate information about the type of mercury used in vaccines and the varying degrees of toxicity and risk.
- Ethylmercury is sometimes present in vaccines. It does not stay in the body for long (about seven days) and is excreted through the gut which makes it less harmful to vital organs.
- Thimerosal is a mercury-containing preservative used in multi-dose vaccines to prevent contamination of virulent bacteria or fungus. Contrary to popular belief, most vaccines in Canada do not contain Thimerosal. School-required vaccines have not contained Thimerosal since 2001.
- Methylmercury is not found in vaccines. This type of mercury can be toxic in high doses and is commonly found in certain kinds of fish. It has a half-life of 45 days, meaning it stays in the system for longer periods, increasing risk of toxicity. For this reason, the federal government regulates methylmercury in the environment to limit exposure.
Time taken for the property to reduce by half

