Chapter 17: Equilibrium and Equilibrium Constants
Enhanced Introductory College Chemistry
by Gregory Anderson; Caryn Fahey; Jackie MacDonald; Adrienne Richards; Samantha Sullivan Sauer; J.R. van Haarlem; and David Wegman;
Chapter Contents
- 17.1 Chemical Reaction Rates
- 17.2 Chemical Equilibria
- 17.3 Equilibrium Constants
- 17.4 Shifting Equilibria: Le Châtelier’s Principle
- 17.5 Equilibrium Calculations
- 17.6 Precipitation and Dissolution
- 17.7 Relative Strengths of Acids and Bases
- 17.8 Real World Examples of Equilibria
- Summary
- Review
Except where otherwise noted, this OER is licensed under CC BY 4.0
Please visit the web version of Enhanced Introductory College Chemistry to access the complete book, interactive activities and ancillary resources.
In this chapter, you will learn about
- Chemical reaction rates
- The dynamic nature of a chemical equilibrium
- Equilibrium constants
- The response of a stressed equilibrium using Le Châtelier’s Principle
- Equilibrium calculations involving changes to the equilibrium
- Equilibrium constants representing solubility, acids and bases
- Real world examples of equilibria in nature
To better support your learning, you should be familiar with the following concepts before starting this chapter:
- Chemical equations
- Reaction stoichiometry
- Molarity
- Solubility
- Reactions of acids and bases
Imagine a beach populated with sunbathers and swimmers. As those basking in the sun get too hot and want to cool off, they head into the surf to swim. As the swimmers tire, they head to the beach to rest. If these two rates of transfer (sunbathers entering the water, swimmers leaving the water) are equal, the number of sunbathers and swimmers would be constant, or at equilibrium, although the identities of the people are constantly changing from sunbather to swimmer and back. An analogous situation occurs in chemical reactions. Reactions can occur in both directions simultaneously (reactants to products and products to reactants) and eventually reach a state of balance.
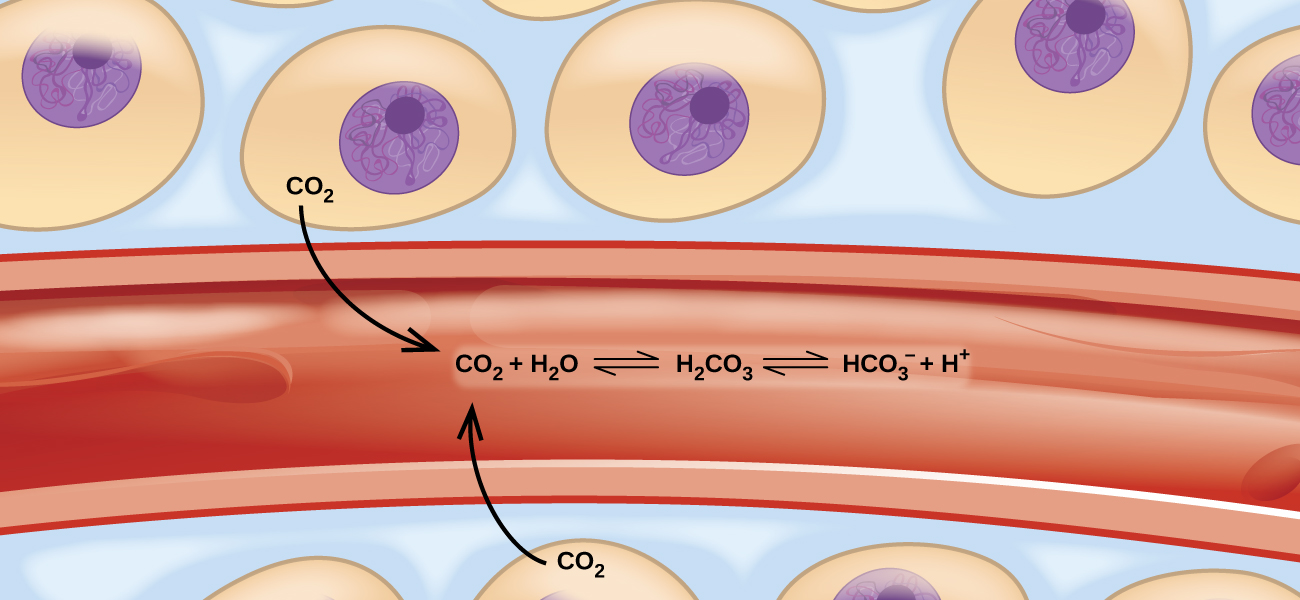
These balanced two-way reactions occur all around and even in us. For example, they occur in our blood, where the reaction between carbon dioxide and water forms carbonic acid (HCO3–) (Figure 17.a). Human physiology is adapted to the amount of ionized products produced by this reaction (HCO3– and H+). In this chapter, you will learn how to predict the position of the balance and the yield of a product of a reaction under specific conditions, how to change a reaction’s conditions to increase or reduce yield, and how to evaluate an equilibrium system’s reaction to disturbances.

