7.2 – Resting, Graded and Action Potential
 |
7.2. Differentiate between resting, graded and action potential with reference to structures of the neuron as well as ion movements and membrane potentials. |
Neurons
The nervous system of the common laboratory fly, Drosophila melanogaster, contains around 100,000 neurons, the same number as a lobster. This number compares to 75 million in the mouse and 300 million in the octopus. A human brain contains around 86 billion neurons. Despite these very different numbers, the nervous systems of these animals control many of the same behaviors—from basic reflexes to more complicated behaviors like finding food and courting mates. The ability of neurons to communicate with each other as well as with other types of cells underlies all of these behaviors.
Most neurons share the same cellular components. But neurons are also highly specialized—different types of neurons have different sizes and shapes that relate to their functional roles.
Parts of a neuron
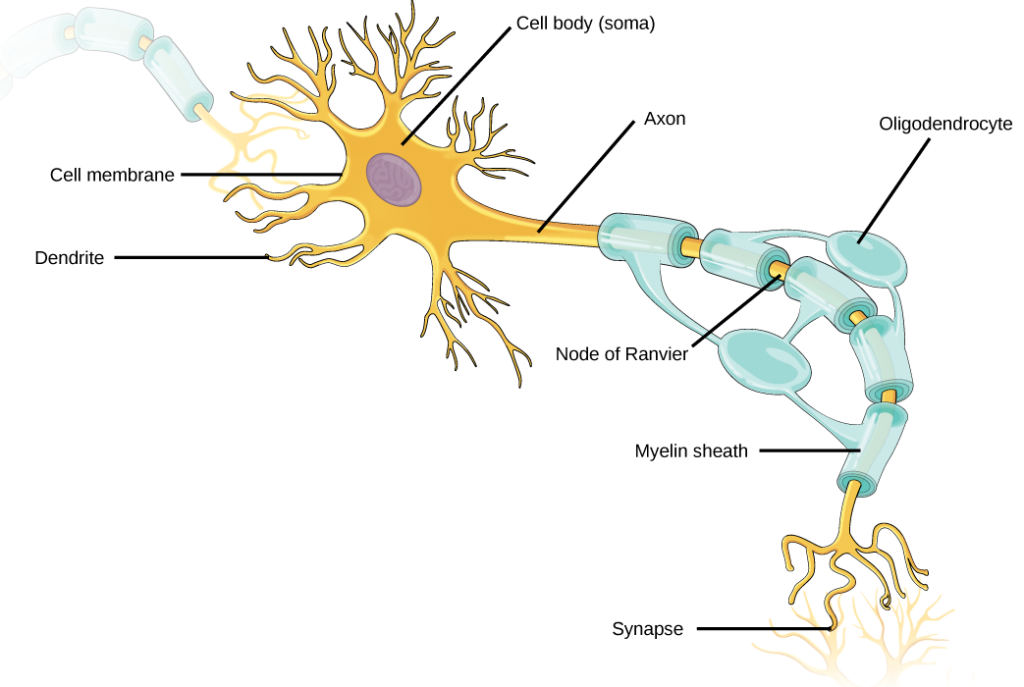
Like other cells, each neuron has a cell body (or soma) that contains a nucleus, smooth and rough endoplasmic reticulum, Golgi apparatus, mitochondria, and other cellular components. Neurons also contain unique structures, illustrated in Figure 7.5 for receiving and sending the electrical signals that make neuronal communication possible. Dendrites are tree-like structures that extend away from the cell body to receive messages from other neurons at specialized junctions called synapses. Although some neurons do not have any dendrites, some types of neurons have multiple dendrites. Dendrites can have small protrusions called dendritic spines, which further increase surface area for possible synaptic connections.
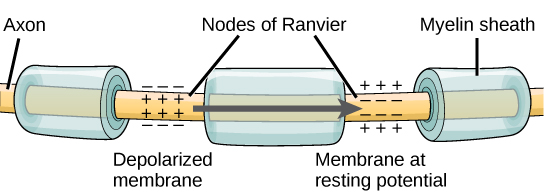
Once a signal is received by the dendrite, it then travels passively to the cell body. The cell body contains a specialized structure, the axon hillock that integrates signals from multiple synapses and serves as a junction between the cell body and an axon. An axon is a tube-like structure that propagates the integrated signal to specialized endings called axon terminals. These terminals in turn synapse on other neurons, muscle, or target organs. Chemicals released at axon terminals allow signals to be communicated to these other cells. Neurons usually have one or two axons, but some neurons, like amacrine cells in the retina, do not contain any axons. Some axons are covered with myelin, which acts as an insulator to minimize dissipation of the electrical signal as it travels down the axon, greatly increasing the speed on conduction. This insulation is important as the axon from a human motor neuron can be as long as a meter—from the base of the spine to the toes. The myelin sheath is not actually part of the neuron. Myelin is produced by glial cells. Along the axon there are periodic gaps in the myelin sheath. These gaps are called nodes of Ranvier and are sites where the signal is “recharged” as it travels along the axon.
It is important to note that a single neuron does not act alone—neuronal communication depends on the connections that neurons make with one another (as well as with other cells, like muscle cells). Dendrites from a single neuron may receive synaptic contact from many other neurons. For example, dendrites from a Purkinje cell in the cerebellum are thought to receive contact from as many as 200,000 other neurons.
 |
Question 7.3
Which of the following statements is false? |
Types of neurons
There are different types of neurons, and the functional role of a given neuron is intimately dependent on its structure. There is an amazing diversity of neuron shapes and sizes found in different parts of the nervous system (and across species), as illustrated by the neurons shown in Figure 7.6.
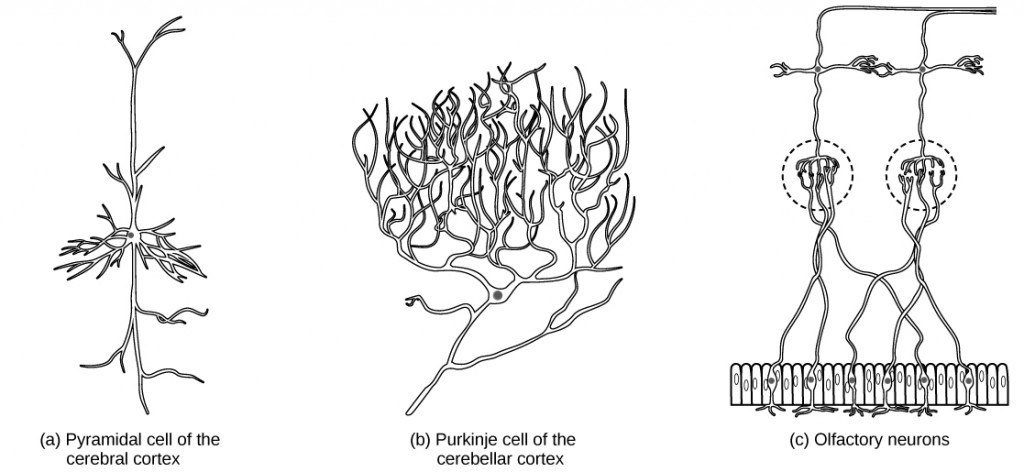
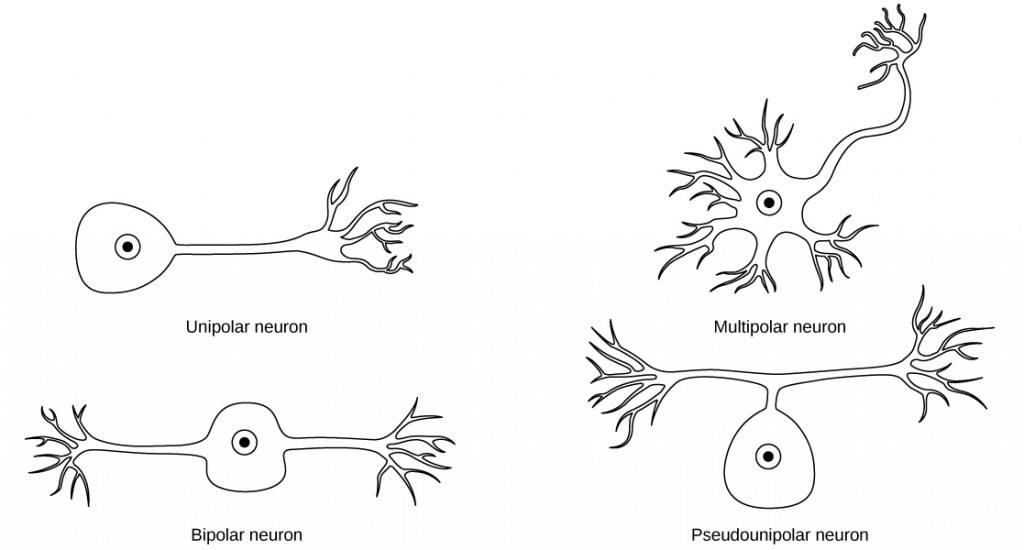
While there are many defined neuron cell subtypes, neurons are broadly divided into four basic types: unipolar, bipolar, multipolar, and pseudounipolar. Figure 7.7 illustrates these four basic neuron types. Unipolar neurons have only one structure that extends away from the soma. These neurons are not found in vertebrates but are found in insects where they stimulate muscles or glands. A bipolar neuron has one axon and one dendrite extending from the soma. An example of a bipolar neuron is a retinal bipolar cell, which receives signals from photoreceptor cells that are sensitive to light and transmits these signals to ganglion cells that carry the signal to the brain. Multipolar neurons are the most common type of neuron. Each multipolar neuron contains one axon and multiple dendrites. Multipolar neurons can be found in the central nervous system (brain and spinal cord). An example of a multipolar neuron is a Purkinje cell in the cerebellum, which has many branching dendrites but only one axon. Pseudounipolar cells share characteristics with both unipolar and bipolar cells. A pseudounipolar cell has a single process that extends from the soma, like a unipolar cell, but this process later branches into two distinct structures, like a bipolar cell. Most sensory neurons are pseudounipolar and have an axon that branches into two extensions: one connected to dendrites that receive sensory information and another that transmits this information to the spinal cord.
Neurogenesis
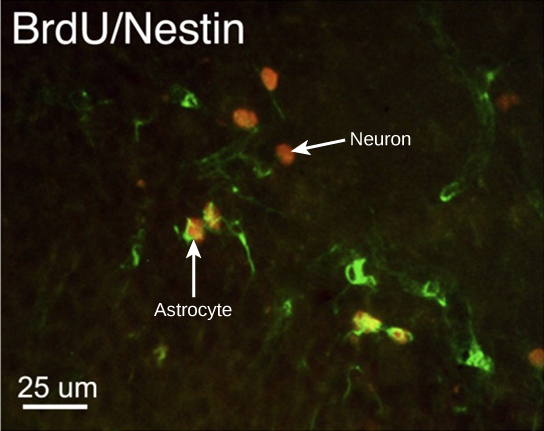
At one time, scientists believed that people were born with all the neurons they would ever have. Research performed during the last few decades indicates that neurogenesis, the birth of new neurons, continues into adulthood. Neurogenesis was first discovered in songbirds that produce new neurons while learning songs. For mammals, new neurons also play an important role in learning: about 1000 new neurons develop in the hippocampus (a brain structure involved in learning and memory) each day. While most of the new neurons will die, researchers found that an increase in the number of surviving new neurons in the hippocampus correlated with how well rats learned a new task. Interestingly, both exercise and some antidepressant medications also promote neurogenesis in the hippocampus. Stress has the opposite effect. While neurogenesis is quite limited compared to regeneration in other tissues, research in this area may lead to new treatments for disorders such as Alzheimer’s, stroke, and epilepsy.
How do scientists identify new neurons? A researcher can inject a compound called bromodeoxyuridine (BrdU) into the brain of an animal. While all cells will be exposed to BrdU, BrdU will only be incorporated into the DNA of newly generated cells that are in S phase. A technique called immunohistochemistry can be used to attach a fluorescent label to the incorporated BrdU, and a researcher can use fluorescent microscopy to visualize the presence of BrdU, and thus new neurons, in brain tissue. Figure 7.8 is a micrograph which shows fluorescently labeled neurons in the hippocampus of a rat.
 |
This site contains more information about neurogenesis, including an interactive laboratory simulation and a video that explains how BrdU labels new cells. |
Nerve impulse transmission within a neuron
For the nervous system to function, neurons must be able to send and receive signals. These signals are possible because each neuron has a charged cellular membrane (a voltage difference between the inside and the outside), and the charge of this membrane can change in response to neurotransmitter molecules released from other neurons and environmental stimuli. To understand how neurons communicate, one must first understand the basis of the baseline or ‘resting’ membrane charge.
Neuronal charged membranes
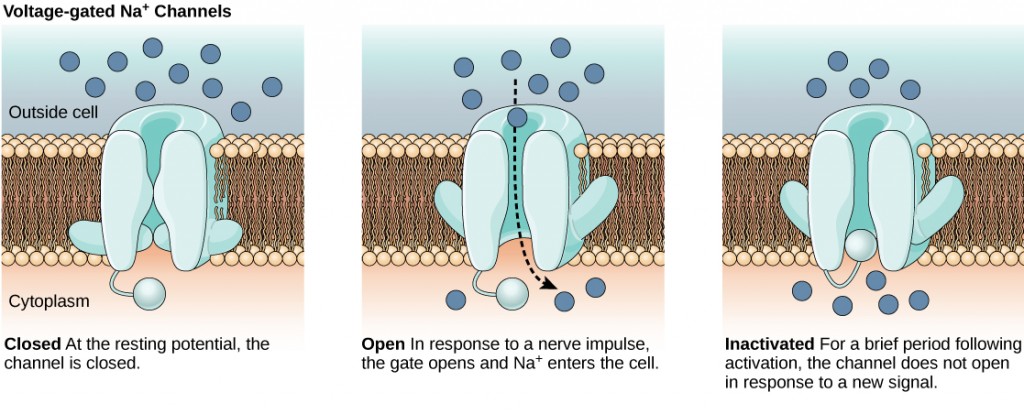
The lipid bilayer membrane that surrounds a neuron is impermeable to charged molecules or ions. To enter or exit the neuron, ions must pass through special proteins called ion channels that span the membrane. Ion channels have different configurations: open, closed, and inactive, as illustrated in Figure 7.9. Some ion channels need to be activated in order to open and allow ions to pass into or out of the cell. These ion channels are sensitive to the environment and can change their shape accordingly. Ion channels that change their structure in response to voltage changes are called voltage-gated ion channels. Voltage-gated ion channels regulate the relative concentrations of different ions inside and outside the cell. The difference in total charge between the inside and outside of the cell is called the membrane potential.
 |
This video discusses the basis of the resting membrane potential. |
Resting membrane potential
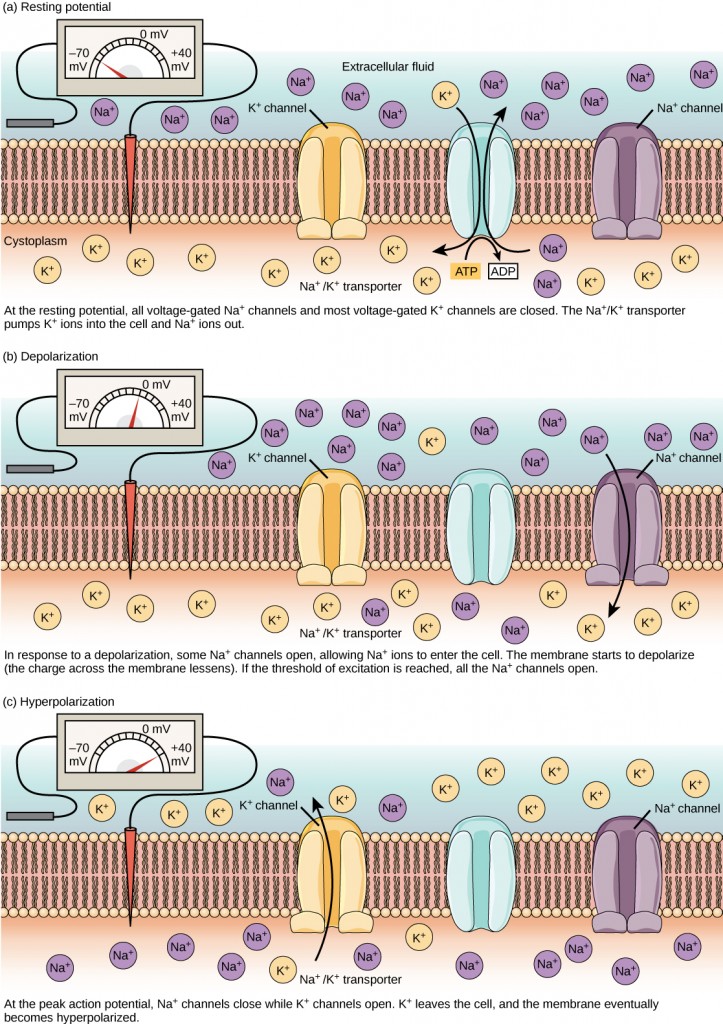
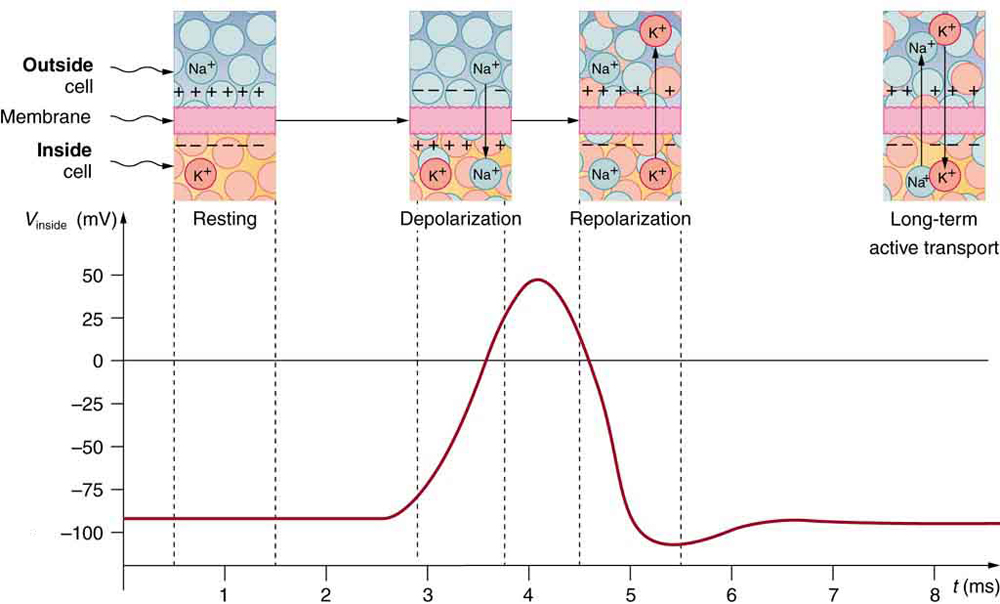
A neuron at rest is negatively charged: the inside of a cell is approximately 70 millivolts more negative than the outside (−70 mV, note that this number varies by neuron type and by species). This voltage is called the resting membrane potential; it is caused by differences in the concentrations of ions inside and outside the cell. If the membrane were equally permeable to all ions, each type of ion would flow across the membrane and the system would reach equilibrium. Because ions cannot simply cross the membrane at will, there are different concentrations of several ions inside and outside the cell, as shown in Table 7.1. The difference in the number of positively charged potassium ions (K+) inside and outside the cell dominates the resting membrane potential (Figure 7.10). When the membrane is at rest, K+ions accumulate inside the cell due to a net movement with the concentration gradient. The negative resting membrane potential is created and maintained by increasing the concentration of cations outside the cell (in the extracellular fluid) relative to inside the cell (in the cytoplasm). The negative charge within the cell is created by the cell membrane being more permeable to potassium ion movement than sodium ion movement. In neurons, potassium ions are maintained at high concentrations within the cell while sodium ions are maintained at high concentrations outside of the cell. The cell possesses potassium and sodium leakage channels that allow the two cations to diffuse down their concentration gradient. However, the neurons have far more potassium leakage channels than sodium leakage channels. Therefore, potassium diffuses out of the cell at a much faster rate than sodium leaks in. Because more cations are leaving the cell than are entering, this causes the interior of the cell to be negatively charged relative to the outside of the cell. The actions of the sodium-potassium pump help to maintain the resting potential, once established. Recall that sodium potassium pumps bring two K+ ions into the cell while removing three Na+ ions per ATP consumed. As more cations are expelled from the cell than taken in, the inside of the cell remains negatively charged relative to the extracellular fluid. It should be noted that calcium ions (Cl–) tend to accumulate outside of the cell because they are repelled by negatively-charged proteins within the cytoplasm.
| Ion Concentration Inside and Outside Neurons | |||
|---|---|---|---|
| Ion | Extracellular concentration (mM) | Intracellular concentration (mM) | Ratio outside/inside |
| Na+ | 145 | 12 | 12 |
| K+ | 4 | 155 | 0.026 |
| Cl− | 120 | 4 | 30 |
| Organic anions (A−) | — | 100 | |
Action potential
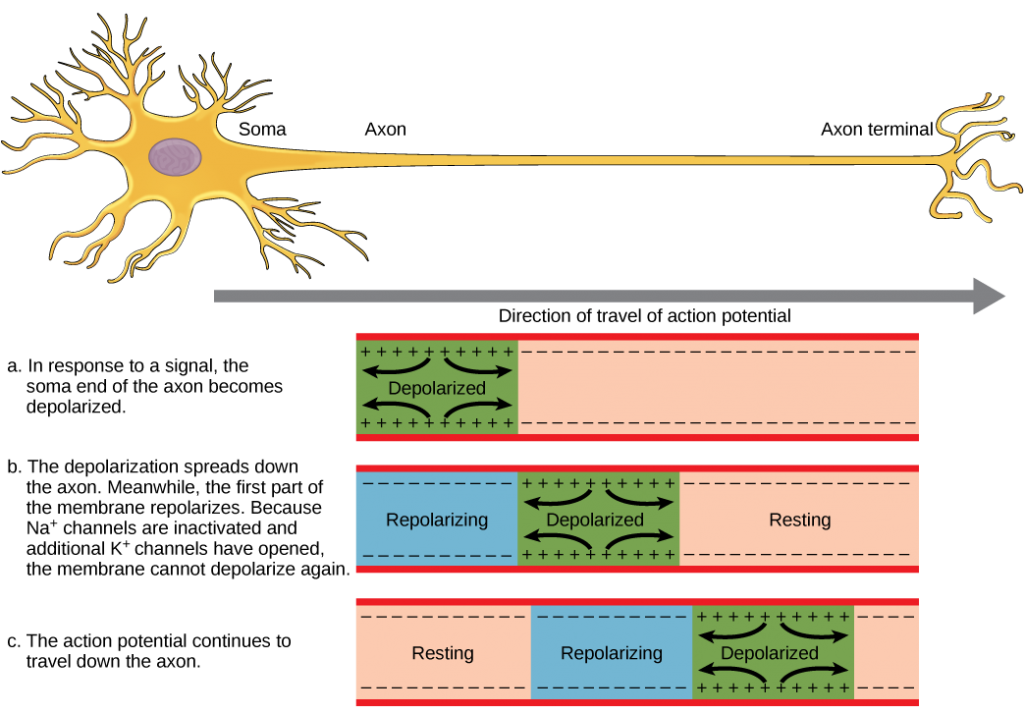
A neuron can receive input from other neurons and, if this input is strong enough, send the signal to downstream neurons. Transmission of a signal between neurons is generally carried by a chemical called a neurotransmitter. Transmission of a signal within a neuron (from dendrite to axon terminal) is carried by a brief reversal of the resting membrane potential called an action potential. When neurotransmitter molecules bind to receptors located on a neuron’s dendrites, ion channels open. At excitatory synapses, this opening allows positive ions to enter the neuron and results in depolarization of the membrane—a decrease in the difference in voltage between the inside and outside of the neuron. A stimulus from a sensory cell or another neuron depolarizes the target neuron to its threshold potential (-55 mV). Na+ channels in the axon hillock open, allowing positive ions to enter the cell (Figure 7.10 and Figure 7.11). Once the sodium channels open, the neuron completely depolarizes to a membrane potential of about +40 mV. Action potentials are considered an “all-or-nothing” event, in that, once the threshold potential is reached, the neuron always completely depolarizes. Once depolarization is complete, the cell must now “reset” its membrane voltage back to the resting potential. To accomplish this, the Na+ channels close and cannot be opened. This begins the neuron’s refractory period, in which it cannot produce another action potential because its sodium channels will not open. At the same time, voltage-gated K+channels open, allowing K+ to leave the cell. As K+ ions leave the cell, the membrane potential once again becomes negative. The diffusion of K+ out of the cell actually hyperpolarizes the cell, in that the membrane potential becomes more negative than the cell’s normal resting potential. At this point, the sodium channels will return to their resting state, meaning they are ready to open again if the membrane potential again exceeds the threshold potential. Eventually, the extra K+ ions diffuse out of the cell through the potassium leakage channels, bringing the cell from its hyperpolarized state, back to its resting membrane potential.
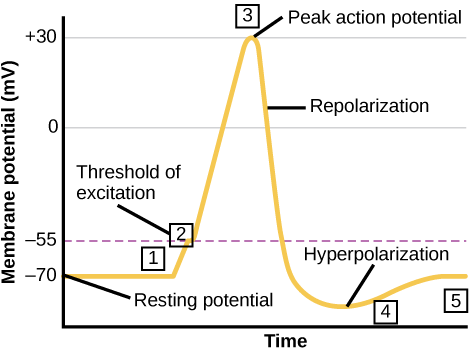
The separation of charge creates a potential difference of 70 to 90 mV across the cell membrane. While this is a small voltage, the resulting electric field () across the only 8-nm-thick membrane is immense (on the order of 11 MV/m!) and has fundamental effects on its structure and permeability. Now, if the exterior of a neuron is taken to be at 0 V, then the interior has a resting potential of about –90 mV. Such voltages are created across the membranes of almost all types of animal cells but are largest in nerve and muscle cells. In fact, fully 25% of the energy used by cells goes toward creating and maintaining these potentials.
Electric currents along the cell membrane are created by any stimulus that changes the membrane’s permeability. The membrane thus temporarily becomes permeable to , which then rushes in, driven both by diffusion and the Coulomb force. This inrush of
first neutralizes the inside membrane, or depolarizes it, and then makes it slightly positive. The depolarization causes the membrane to again become impermeable to
, and the movement of
quickly returns the cell to its resting potential, or repolarizes it. This sequence of events results in a voltage pulse called the action potential (Figure 7.12). Only small fractions of the ions move so that the cell can fire many hundreds of times without depleting the excess concentrations of
and
. Eventually, the cell must replenish these ions to maintain the concentration differences that create bioelectricity. This sodium-potassium pump is an example of active transport, wherein cell energy is used to move ions across membranes against diffusion gradients and the Coulomb force.
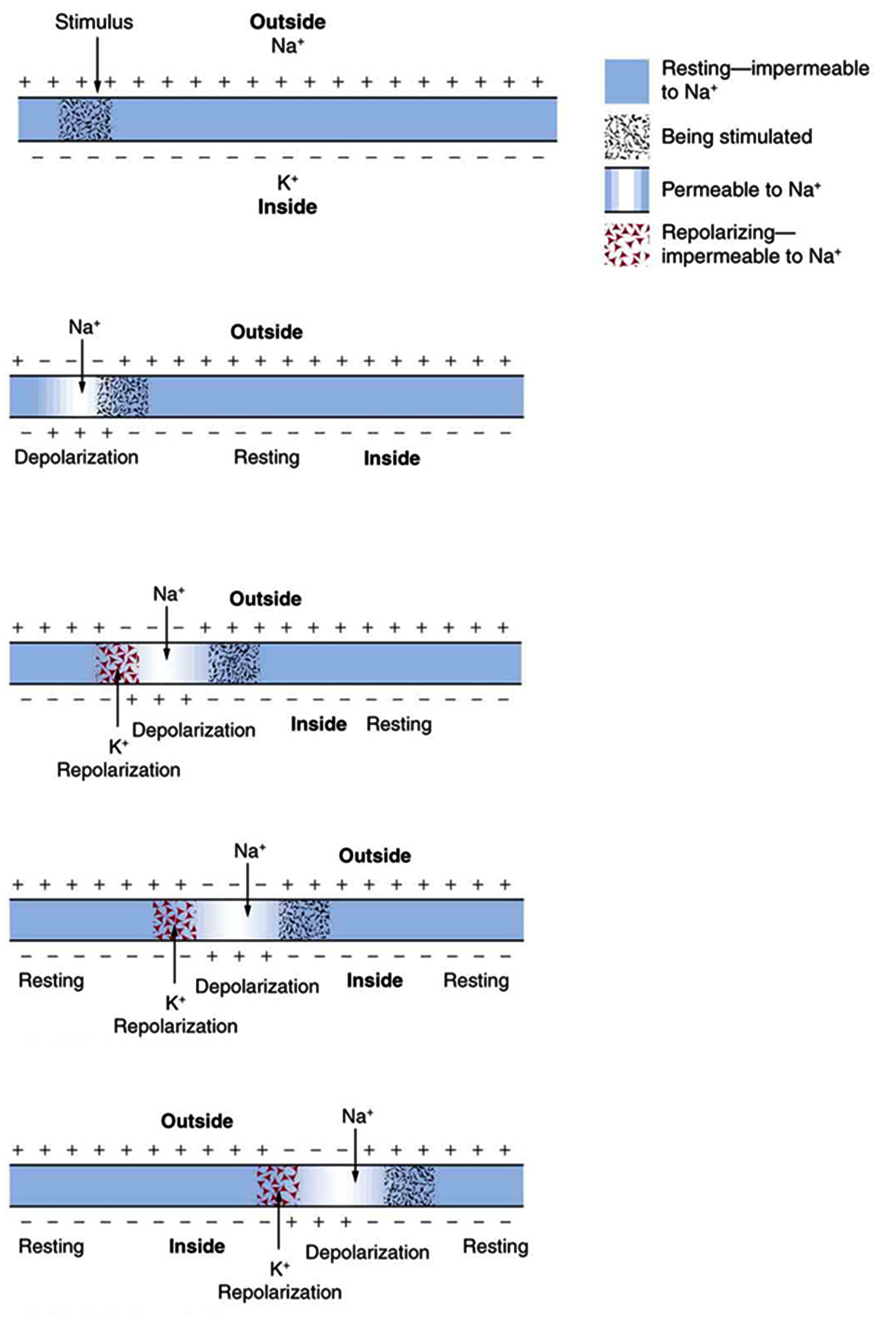
The action potential is a voltage pulse at one location on a cell membrane. How does it get transmitted along the cell membrane, and in particular down an axon, as a nerve impulse? The answer is that the changing voltage and electric fields affect the permeability of the adjacent cell membrane so that the same process takes place there. The adjacent membrane depolarizes, affecting the membrane further down, and so on, as illustrated in Figure 7.6. Thus the action potential stimulated at one location triggers a nerve impulse that moves slowly (about 1 m/s) along the cell membrane.
 |
Question 7.4
Potassium channel blockers, such as amiodarone and procainamide, which are used to treat abnormal electrical activity in the heart, called cardiac dysrhythmia, impede the movement of K+ through voltage-gated K+ channels. Which part of the action potential would you expect potassium channels to affect? Explain why. |
 |
This video presents an overview of an action potential. |
Myelin and the propagation of the action potential
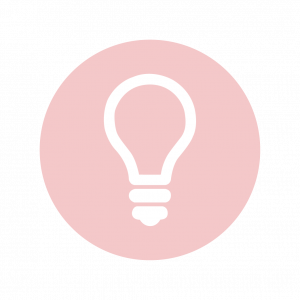
For an action potential to communicate information to another neuron, it must travel along the axon and reach the axon terminals where it can initiate neurotransmitter release. The speed of conduction of an action potential along an axon is influenced by both the diameter of the axon and the axon’s resistance to current leak. Myelin acts as an insulator that prevents current from leaving the axon; this increases the speed of action potential conduction. In demyelinating diseases like multiple sclerosis, action potential conduction slows because the current leaks from previously insulated axon areas. The nodes of Ranvier, illustrated in Figure 7.15 are gaps in the myelin sheath along the axon. These unmyelinated spaces are about one micrometer long and contain voltage-gated Na+ and K+ channels. The flow of ions through these channels, particularly the Na+ channels, regenerates the action potential over and over again along the axon. This ‘jumping’ of the action potential from one node to the next is called saltatory conduction. If nodes of Ranvier were not present along an axon, the action potential would propagate very slowly since Na+ and K+ channels would have to continuously regenerate action potentials at every point along the axon instead of at specific points. Nodes of Ranvier also save energy for the neuron since the channels only need to be present at the nodes and not along the entire axon.
Figure 7.16 shows an enlarged view of an axon having myelin sheaths characteristically separated by unmyelinated gaps (called nodes of Ranvier). This arrangement gives the axon a number of interesting properties. Since myelin is an insulator, it prevents signals from jumping between adjacent nerves (crosstalk). Additionally, the myelinated regions transmit electrical signals at a very high speed, as an ordinary conductor or resistor would. There is no action potential in the myelinated regions so that no cell energy is used in them. There is an signal loss in the myelin, but the signal is regenerated in the gaps, where the voltage pulse triggers the action potential at full voltage. So a myelinated axon transmits a nerve impulse faster, with less energy consumption, and is better protected from cross talk than an unmyelinated one. Not all axons are myelinated so that crosstalk and slow signal transmission are a characteristic of the normal operation of these axons, another variable in the nervous system.
The degeneration or destruction of the myelin sheaths that surround the nerve fibers impairs signal transmission and can lead to numerous neurological effects. One of the most prominent of these diseases comes from the body’s own immune system attacking the myelin in the central nervous system—multiple sclerosis. MS symptoms include fatigue, vision problems, weakness of arms and legs, loss of balance, and tingling or numbness in one’s extremities (neuropathy). It is more apt to strike younger adults, especially females. Causes might come from infection, environmental or geographic effects, or genetics. At the moment there is no known cure for MS.
Most animal cells can fire or create their own action potential. Muscle cells contract when they fire and are often induced to do so by a nerve impulse. In fact, nerve and muscle cells are physiologically similar, and there are even hybrid cells, such as in the heart, that have characteristics of both nerves and muscles. Some animals, like the infamous electric eel (Figure 7.17) use muscles ganged so that their voltages add in order to create a shock great enough to stun prey.
 |
Question 7.5
Which of the following statements is false? |
 |
Question 7.6
Neurons contain ________, which can receive signals from other neurons. |
 |
Question 7.7
A(n) ________ neuron has one axon and one dendrite extending directly from the cell body. |
 |
Question 7.8
Glia that provide myelin for neurons in the brain are called ________. |
 |
Question 7.9
How are neurons similar to other cells? How are they unique? |
 |
Question 7.10
Compare and contrast resting, graded and action potential? In your answer, make sure you have included channels and voltage reference as well as relevant structures of the neurons. Once you have come up with an answer, give it to another student to review. Based on the review by your peer, is there something you need to work on in terms of your understanding of the resting, graded and action potential. |
 |
Question 7.11
Multiple sclerosis causes demyelination of axons in the brain and spinal cord. Why is this problematic? |