43 Case 1-2020: A 62-Year-Old Woman with Early Breast Cancer during the Covid-19 Pandemic
Case 22-2020: A 62-Year-Old Woman with Early Breast Cancer during the Covid-19 Pandemic.
Laura M. Spring, Michelle C. Specht, Rachel B. Jimenez, Steven J. Isakoff, Gary X. Wang, Amy Ly, Jennifer A. Shin, Aditya Bardia, and Beverly Moy.
Case Summary1
A 62-year-old Ashkenazi Jewish female presented during the COVID-19 pandemic with a palpable left breast mass. Past history included fibroadenoma excision and asthma, with no family history of breast or ovarian cancer. Imaging revealed a 3 cm irregular lesion, and biopsy confirmed a moderately differentiated invasive tumor, ER/PR positive and HER2 negative. A 21-gene recurrence assay showed intermediate risk, and she was treated per clinical guidelines with follow-up planned.
Learning Objectives
- Analyze immunohistochemistry (IHC) results, including estrogen receptor (ER), progesterone receptor (PR), and HER2 status, to guide targeted therapy decisions.
- Apply the Oncotype DX 21-gene recurrence score to predict recurrence risk and guide personalized treatment decisions.
- Understand the significance of proliferation markers (e.g., Ki-67) in assessing tumor aggressiveness, even if not reported in this case.
Clinical History1
- Age: 62 years old
- Sex: Female
- Ethnicity: Ashkenazi Jewish
Presenting Symptoms1
The patient discovered a palpable mass in her left breast
Medical History1
- Asthma
- She had fibroadenoma of the left breast (excised 30 years ago).
- No family history of breast or ovarian cancer.
- Menarche at 12 years; menopause at 54 years; no hormone replacement therapy.
- She denied pain, nipple discharge, or systemic symptoms.
Drug/Allergies
- Not specified.
Physical Examination1
A firm mass measuring approximately 3 cm was palpable in the left breast, with no palpable axillary lymphadenopathy.
Investigations
Imaging Studies1
- Mammography: Irregular mass with spiculated margins in the left breast (Figures 1A-C).
- Ultrasound: Solid, irregular mass 3.1 × 1.5 × 1.2 cm; normal left axillary lymph nodes (Figure 1D).
- Core-needle biopsy: Tissue obtained for histology and immunohistochemistry. Performed under ultrasound guidance (Figure 1E).
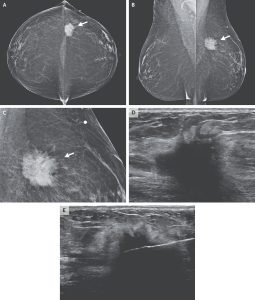
Figure 1: Bilateral mammograms taken in the craniocaudal and mediolateral oblique views (Panels A and B, respectively) reveal a mass in the left breast beneath the skin marker (white arrows on panel A and B). On magnified view (Panel C), the lesion appears irregular and spiculated (white arrow on Panel C). Ultrasound imaging (Panel D) demonstrates a solid, irregular mass measuring 3.1 × 1.5 × 1.2 cm. An image obtained during an ultrasound-guided core-needle biopsy (Panel E) shows the needle accurately positioned within the mass.1
Table 1: Pathological Investigations1,2
|
Test Name |
Patient’s Result |
Reference Range |
Interpretation |
|---|---|---|---|
|
Core-needle biopsy |
Invasive ductal carcinoma, grade 2 |
N/A |
Confirms malignant breast lesion. |
|
ER status |
Strongly positive |
Negative/Positive |
The tumor is estrogen receptor positive. |
|
PR status |
Strongly positive |
Negative/Positive |
The tumor is progesterone receptor positive. |
|
HER2 status |
Equivocal on IHC; negative by FISH |
Negative/Positive |
No HER2 amplification. |
|
Tumor size |
3.1 × 1.5 × 1.2 cm |
N/A |
Clinical stage T2. |
|
Oncotype DX 21-gene recurrence score |
24 |
0–100 |
Intermediate risk; may derive limited benefit from chemotherapy. |
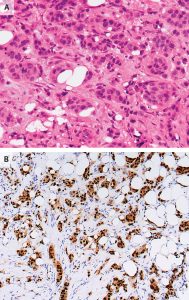
Figure 2:Histologic and immunohistochemical analysis of invasive ductal carcinoma.
Panel A (H&E staining):Hematoxylin and eosin staining of the core-needle biopsy specimen shows invasive ductal carcinoma, characterized by irregular nests and cords of malignant epithelial cells infiltrating the fibrous stroma. The tumor cells exhibit pleomorphic nuclei, increased nuclear-to-cytoplasmic ratio, and mitotic activity, confirming their malignant nature.
Panel B (IHC staining):Immunohistochemical staining demonstrates strong, diffuse nuclear positivity for both estrogen receptors (ER) and progesterone receptors (PR). Tumor cell nuclei appear brown due to chromogenic staining (DAB), indicating positive receptor expression, while surrounding non-tumor stromal cells remain blue with hematoxylin counterstaining, confirming hormone receptor–positivity (HR+).1
Clinical Assay Design and Testing
Principles of Oncotype DX 21-Gene Assay1,2
The Oncotype DX assay evaluates the expression of 21 genes (16 cancer-related and 5 reference genes) in tumor tissue using reverse transcription polymerase chain reaction (RT-PCR). The results are combined into a recurrence score (RS) ranging from 0 to 100. The RS predicts the risk of distant recurrence and potential chemotherapy benefit.
Test Procedure
- Sample Preparation: RNA is extracted from formalin-fixed, paraffin-embedded tumor tissue obtained via core-needle biopsy.
- RT-PCR Quantification: Expression levels of the 21 genes are measured.
- Recurrence Score Calculation: Expression levels of cancer-related genes are normalized to reference genes; weighted coefficients generate the RS.
Clinical Interpretation2
- RS <18: Low risk; chemotherapy generally not indicated.
- RS 18–30: Intermediate risk; chemotherapy may be omitted in postmenopausal women.
- RS ≥31: High risk; chemotherapy recommended.
Proliferation Markers5 (e.g., Ki-67)
- Ki-67 measures actively dividing tumor cells.
- High Ki-67: Rapid tumor growth, higher recurrence risk, may support chemotherapy.
- Low Ki-67: Slower growth, often seen in HR+, HER2-negative; endocrine therapy sufficient.
- Though not reported here, Ki-67 complements ER/PR/HER2 data for therapy decisions.
Table 2: TNM Staging System for Breast Cancer3
|
Category |
Code |
Description |
Size / Extent |
|---|---|---|---|
|
Tumor (T) |
T0 |
No primary tumor |
— |
|
|
Tis |
Carcinoma in situ |
— |
|
|
T1 |
Small tumor |
≤ 2 cm |
|
|
T2 |
Medium tumor |
> 2 cm to ≤ 5 cm |
|
|
T3 |
Large tumor |
> 5 cm |
|
|
T4 |
Tumor of any size invading the chest wall or skin |
Variable |
|
Nodes (N) |
N0 |
No lymph node involvement |
— |
|
|
N1 |
Cancer in 1–3 axillary lymph nodes |
— |
|
|
N2 |
Cancer in 4–9 axillary lymph nodes |
— |
|
|
N3 |
Cancer in ≥10 axillary or supraclavicular nodes |
— |
|
Metastasis (M) |
M0 |
No distant metastasis |
— |
|
|
M1 |
Distant metastasis present (spread to other organs) |
— |
Note: Units are in centimeters (cm)
4. Guiding questions leading to diagnosis
Question 3: Based on the Immunohistochemistry results, what is the hormone receptor (HR) status of the tumor, and how does it influence the choice of endocrine therapy?
Question 4: How is human epidermal growth factor receptor 2 (HER2) status assessed by immunohistochemistry (IHC) and Fluorescence in situ hybridization (FISH), and why is it important for treatment planning in early-stage breast cancer?
Question 5: Proliferation markers such as Ki-67 are often used in breast cancer evaluation to assess tumor aggressiveness. Although this case report did not include Ki-67 results, what is the clinical significance of proliferation markers, and how could such information complement the immunohistochemistry (IHC) findings if it were available?
** For answers please check the next chapter**
Appendix I: Medical Terminology6
|
Term |
Definition |
|---|---|
|
HR-positive |
Hormone receptor–positive; tumor cells express estrogen (ER) and/or progesterone (PR) receptors, indicating likely response to endocrine therapy. |
|
HER2-negative |
Tumor cells do not overexpress the human epidermal growth factor receptor 2 protein. |
|
Invasive ductal carcinoma (IDC) |
The most common type of breast cancer originating from the milk ducts and invading surrounding breast tissue. |
|
Recurrence Score (RS) |
A score (0–100) derived from the Oncotype DX 21-gene assay that predicts the risk of distant recurrence and chemotherapy benefit in HR+/HER2– breast cancer. |
|
Neoadjuvant therapy |
Treatment given before surgery to shrink a tumor, often including chemotherapy or endocrine therapy. |
|
Core-needle biopsy |
A procedure using a hollow needle to extract tissue from a breast mass for histopathologic examination. |
|
Mammography |
Radiographic imaging of the breast is used to detect masses, calcifications, or other abnormalities. |
|
Ultrasonography (US) |
Imaging modality using sound waves to evaluate breast lesions, differentiating solid from cystic masses. |
|
Tumor Grade |
Histologic assessment of how much tumor cells resemble normal cells; higher grade indicates more aggressive disease. |
|
TNM Staging |
The Tumor-Node-Metastasis system is used to classify the extent of breast cancer. |
|
ER/PR positivity |
Indicates tumor cells have receptors for estrogen or progesterone, guiding endocrine therapy selection. |
|
IHC |
Immunohistochemistry is used to detect specific proteins such as ER, PR, or HER2 in tissue samples |
|
FISH |
Fluorescence in situ hybridization detects gene amplification, such as HER2 |
References
- Spring, L. M., Specht, M. C., Jimenez, R. B., Isakoff, S. J., Wang, G. X., Ly, A., Shin, J. A., Bardia, A., & Moy, B. (2020). Clinical application of the 21-gene assay in early-stage breast cancer. New England Journal of Medicine, 383(3), 262–272. https://doi.org/10.1056/NEJMcpc2002422
- Kelly, C. M., Krishnamurthy, S., Bianchini, G., Litton, J. K., Gonzalez-Angulo, A. M., Hortobagyi, G. N., & Pusztai, L. (2010). Utility of Oncotype DX risk estimates in clinically intermediate-risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer, https://acsjournals.onlinelibrary.wiley.com/doi/pdfdirect/10.1002/cncr.25269
- American Cancer Society. (2024). TNM staging for breast cancer. Retrieved from.Stages of Breast Cancer | Understand Breast Cancer Staging | American Cancer Society
- Iqbal, N., & Iqbal, N. (2014). Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Molecular Biology International, 2014, Article 852748. https://doi.org/10.1155/2014/852748
- Louis, D. M., Nair, L. M., Vallonthaiel, A. G., Narmadha, M. P., & Vijaykumar, D. K. (2022). Ki-67: a promising prognostic marker in early breast cancer—a review article. Indian Journal of Surgical Oncology, 14(1), 122–127. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9986372/
- National Cancer Institute. (2024). Hormone receptor-positive. In NCI Dictionary of Cancer Terms. https://www.cancer.gov/publications/dictionaries/cancer-terms
A fibroadenoma is a non-cancerous, firm lump in the breast.
A lab method that uses antibodies to detect specific proteins in tissue samples.
Lymphadenopathy or adenopathy is a disease of the lymph nodes, in which they are abnormal in size or consistency.
https://en.wikipedia.org/wiki/Lymphadenopathy

