15 Case 2024: Hereditary Hemochromatosis Caused by a C282Y/H63D Mutation in the HFE Gene.
Hereditary Hemochromatosis Caused by a C282Y/H63D Mutation in the HFE Gene: Dongdong Li, Jinfeng Li, Hongkun Zhang, Qiuyu Zhu, Teng Wang, Wen Zhao, Shousong Zhao, Wei Li.
Clinical case summary1
A 68-year-old Chinese male was admitted with persistent hyperbilirubinemia for over 3 months. He had a 40-year history of smoking and daily alcohol consumption. On examination, he had bronze skin, hyperpigmentation in skin folds, and slight scleral jaundice. Laboratory tests revealed elevated indirect bilirubin, markedly high serum ferritin, and abnormal transferrin saturation. MRI showed hypointense liver parenchyma black liver and splenomegaly. Liver biopsy confirmed grade 4+ iron deposition with fibrosis and inflammation. Genetic testing revealed a heterozygous C282Y/H63D mutation in the HFE gene. Therapeutic phlebotomy over 2 years normalized iron indices, improved skin pigmentation, and alleviated fatigue.
Learning Objectives
- Identify clinical and laboratory findings of hereditary hemochromatosis (HH).
- Recognize the significance of imaging and liver biopsy in diagnosis.
- Understand the role of HFE gene mutations in iron overload.
Clinical History1
- Age: 68 years
- Sex: Male
- Ethnicity: Chinese
Medical History1
- A 40-year history of smoking takes 10 cigarettes/day, and alcohol intake is about 100 g/day.
- No history of oral iron supplementation or blood transfusions.
- No known family history of HH (parents are deceased).
Presenting symptoms1
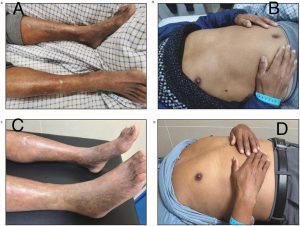
- Bronze skin and hyperpigmentation in skin folds
- Slight scleral jaundice
- Fatigue
- Occasional palpitations and chest discomfort.
Physical Examination1
- Widespread bronze pigmentation of skin and skin folds, Mild scleral jaundice
- No palpable hepatosplenomegaly
- Vital signs are normal.
Laboratory Investigations1
| Test (Full Name) | Reference Value | Pretreatment | Posttreatment |
| Routine Blood Panel | |||
| White Blood Cell Count(×10⁹/L) | 4.0–10.0 | 7.72 | 8.78 |
| Neutrophil Count (×10⁹/L) | 2.0–7.0 | 4.85 | 5.79 |
| Red Blood Cell Count (×10¹²/L) | ♂ 4.3–5.8 / ♀ 3.8–5.1 | 4.05 | 3.87 |
| Hemoglobin (g/L) | ♂ 130–175 / ♀ 115–155 | 138 | 126 |
| Routine Biochemical Tests | |||
| Alanine Aminotransferase (U/L) | < 40 | 41 | 12 |
| Aspartate Aminotransferase (U/L) | < 35 | 46 | 19 |
| Total Bilirubin (μmol/L) | 3.4–20.5 | 73.3 | 15.6 |
| Direct Bilirubin (μmol/L) | 0–6.8 | 10.7 | 7.2 |
| Indirect Bilirubin (μmol/L) | 3.4–13.7 | 62.6 | 8.4 |
| Total Protein (g/L) | 65–85 | 71.4 | 75.1 |
| Albumin (g/L) | 35–52 | 45.6 | 42.4 |
| Globulin (g/L) | 20–35 | 25.8 | 32.9 |
| Glucose (mmol/L) | 3.9–6.1 | 3.94 | 4.25 |
| Total Cholesterol (mmol/L) | < 5.2 | 1.91 | 2.35 |
| Triglycerides (mmol/L) | < 1.7 | 0.71 | 1.05 |
| Uric Acid (μmol/L) | ♂ 208–428 / ♀ 155–357 | 167 | 188 |
| Serum Iron Index | |||
| Serum Iron (μmol/L) | 10–30 | 40 | 24.6 |
| Serum Ferritin (μg/L) | ♂ 30–400 / ♀ 15–150 | 1550 | 454 |
| Transferrin (mg/dL) | 200–360 | 136 | 226 |
| Transferrin Saturation (%) | 20–45 | 97.5 | 55.1 |
Table 1
Pre-treatment Findings from the table
Elevated total and indirect bilirubin
Mildly elevated liver enzymes (ALT and AST)
Markedly elevated serum ferritin and high transferrin saturation (97.5%), suggesting iron overload
Mildly elevated serum iron.
Post-treatment Findings from the table
Liver enzymes and bilirubin normalized
Serum ferritin, iron, and transferrin saturation returned to normal
Overall improvement in biochemical and hematologic parameters.
Viral hepatitis, autoimmune hepatitis, tumor markers, Ham test and Coombs test were all negative
Imaging1
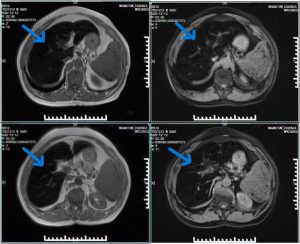
- MRI liver: Diffuse low T1/T2 signal intensity consistent with iron overload (“black liver”)
Ultrasound: Normal liver shape, gallstones, splenomegaly; no Budd-Chiari syndrome
ECG: Sinus tachycardia, right bundle branch block, atrial fibrillation
Echocardiography: Left heart enlargement
Liver biopsy1
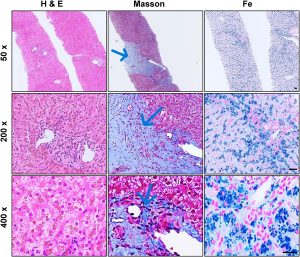
Hematoxylin and Eosin (H&E staining showed preserved lobular structure with tan pigment granules in hepatocytes.
Masson staining showed central vein fibrosis with occasional bridging fibrosis.
Prussian blue showed Grade 4+ iron deposition in hepatocytes, sparse in Kupffer cells and plasma cells.
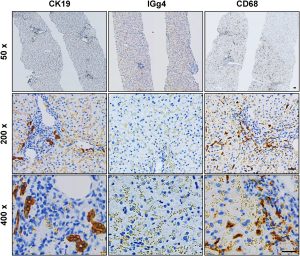
Immunohistochemistry1: CK19+ cholangiocytes, IgG4+ hepatocytes, activated Kupffer cells, lymphocytes, and plasma cells in lesions. indicating inflammation and fibrosis due to iron overload.
Genetic Testing1
Genetic testing of the HFE gene revealed heterozygosity with two mutations, C282Y and H63D. The H63D variant was confirmed as a missense mutation (c.187C>G, p.His63Asp).
Guiding Questions and Answers
Question 1: Based on chief complaints, medical history, and physical examination, what initial diagnoses can we suspect?
Question 2: What is the Underlying Genetic Cause?
Question 3: To confirm the diagnosis, what laboratory investigations should be done, and how would you interpret the results in this case?
Question 4: If this condition remains untreated, what complications might arise?
** For answers please check the next chapter **
Medical terminology
- Hereditary Hemochromatosis (HH): Autosomal recessive disorder causing iron overload
- HFE gene: Regulates iron absorption
- Transferrin Saturation (TSAT): % of transferrin bound to iron
- Serum Ferritin (SF): Iron storage marker
- Phlebotomy: Therapeutic blood removal to reduce iron load
- Hyperbilirubinemia: Elevated levels of bilirubin in the blood
- Transferrin: Blood protein that transports iron throughout the body
- Hepcidin: Liver hormone that regulates iron absorption in the intestines
- Hepatocytes: Main functional cells of the liver that perform metabolic functions
- Kupffer cells: Specialized immune cells (macrophages) located in the liver
- Fibrosis: Formation of excess fibrous connective tissue in response to injury or damage
- Cirrhosis: Advanced stage of liver fibrosis with scarring and impaired liver function
- Scleral jaundice: Yellowing of the white part of the eyes due to elevated bilirubin
- Splenomegaly: Abnormal enlargement of the spleen
- Hepatomegaly: Abnormal enlargement of the liver
- Cardiomyopathy: Disease of the heart muscle affecting its ability to pump blood
- Autosomal recessive: Inheritance pattern requiring two copies of a mutated gene (one from each parent) to express the disease
- Indirect bilirubin (IB): Unconjugated bilirubin; elevated levels may indicate hemolysis or liver dysfunction
- Direct bilirubin (DB): Conjugated bilirubin; elevated levels suggest bile duct obstruction or liver disease
- Prussian blue staining: Histological staining technique used to detect iron deposits in tissues
- H&E staining (Hematoxylin and Eosin): Standard histological staining technique that shows tissue structure
- Masson staining: Histological staining technique used to identify fibrosis and collagen deposition
- Modified Scheuer score: Grading system for assessing liver inflammation (G) and fibrosis (S)
- Bronze skin pigmentation: Characteristic brownish or bronze discoloration of skin due to iron and melanin deposition
- MRI (Magnetic Resonance Imaging): Imaging technique; in hemochromatosis shows “black liver” due to iron deposition
- ALT (Alanine Aminotransferase): Liver enzyme; elevated levels indicate liver damage
- AST (Aspartate Aminotransferase): Liver enzyme; elevated levels indicate liver or heart damage
References
- Li, D., Li, J., Zhang, H., Zhu, Q., Wang, T., Zhao, W., Zhao, S., & Li, W. (2024). Hereditary hemochromatosis caused by a C282Y/H63D mutation in the HFE gene: A case report. Heliyon, 10, e28046. https://doi.org/10.1016/j.heliyon.2024.e28046
- Bacon, B. R., Adams, P. C., Kowdley, K. V., Powell, L. W., & Tavill, A. S. (2011). Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology, 54(1), 328–343. https://doi.org/10.1002/hep.24330
- Zoller, H., Fauvert, J.-F., Girelli, D., & European Association for the Study of the Liver. (2022). EASL Clinical Practice Guidelines on haemochromatosis. Journal of Hepatology, 77(2), 479–502. https://www.journal-of-hepatology.eu/article/S0168-8278(22)00211-2/fulltext
- Szczerbinska, A., Kasztelan‑Szczerbińska, B., Rycyk‑Bojarzynska, A., Kocki, J., & Cichoz‑Lach, H. (2024). Hemochromatosis—How Not to Overlook and Properly Manage “Iron People”. Journal of Clinical Medicine, 13(13), 3660. https://doi.org/10.3390/jcm13133660
- Delatycki, M. B., & Allen, K. J. (2024). Population Screening for Hereditary Haemochromatosis — Should It Be Carried Out, and If So, How? Genes, 15(8), 967. https://doi.org/10.3390/genes15080967
- Agrawal, A., El Dahdah, J., Haroun, E., Arockiam, A. D., Safdar, A., Sorathia, S., Dong, T., Griffin, B., & Wang, T. K. M. (2025). A Contemporary Review of Clinical Manifestations, Evaluation, and Management of Cardiac Complications of Iron Overload. Hearts, 6(3), 17. https://doi.org/10.3390/hearts6030017
Elevated levels of bilirubin in the blood
Blood protein that transports iron throughout the body
the enlargement of the spleen.
Formation of excess fibrous connective tissue in response to injury or damage
Therapeutic blood removal to reduce iron load
Autosomal recessive disorder causing iron overload
Yellowing of the white part of the eyes due to elevated bilirubin
It is a rare condition caused by blockage of the hepatic veins, which are the veins that drain blood out of the liver.
Specialized immune cells (macrophages) located in the liver
Regulates iron absorption

