Optical Tweezers
Procedure
4.1 Sample Preparation
Be patient, be careful, and take your time making the sample. Repeated mistakes making the sample is the main reason students fail to collect data within the time allotted. Make sure you’ve watched the recording of this experiment so that you’ve seen a sample prepared before you attend the lab.
Figure 5: The microscope slide, double-sided tape, and slide cover used to make a channel for the bead solution. Use a razorblade to remove the excess tape when you’re done.
- Open the laser enclosure and make sure that you have space to insert the sample slide without bumping/scratching the objective or condenser lenses.
- Make sure the table clamp is vacuum-mounted to the table and secure. Place a microscope slide securely in the clamp.
- Use two pieces of double-sided tap to make a channel approximately 2 mm wide, as shown in Figure 5. Use a toothpick to push on the tape and make sure it is firmly attached to the microscope slide.
- Place a slide cover so that it is centered on the channel. Use the toothpick to make sure that the slide cover is firmly attached to the tape.
- CAREFULLY use the razor blade to remove the excess tape. If you would rather not handle the razorblade, ask your TA for help.
- Use a fresh tip for the micropipette and take a SMALL sample of the beads from the provided bottle. For best results, shake the bottle for a few minutes first, then hold the micropipette near the edge of the bottle. You need less than half of the plastic tip of the micropipette of solution.
- Inject the solution into the channel you made on the microscope slide. You will likely want to brace your hand and be very steady for this part. Try to watch the solution as it fills the channel and stop adding solution when the channel is full.
- Use another toothpick to get a small dab of vacuum grease. Use the grease to seal the two ends of the channel. Try not to make much of a mess – a small dab of well-placed grease will work better than a large, haphazardly placed glob.
- Consider having the TA or technician have a look once you’re done.
- If there is any remaining solution in the pipette, then return it to the bottle.
4.2 Finding & Trapping a Bead
- Place a dab of immersion oil on the slide cover, flip it over, and place it in the microscope slide holder in the laser enclosure.
- Lower the slide holder until the immersion oil wets to the objective lens.
- Use the manual x and y micrometer translation stage to bring the tape-edge of the channel over the objective lens. While watching the camera viewer, use the micrometer translation in the z axis to bring the tape edge into focus. The sample is now near the ideal distance from the objective.
- Use the x and y translation to move to the center of the channel, then make small adjustments in the z axis to find beads in the sample.
- The beads should be moving in a very Brownian way – random motion in all three axes. If all of the beads you see are travelling in the same direction, then it’s likely that your sample is leaking – you need a new one.
- Close the enclosure and turn on the laser. Start out with a current of about 200 mA. Use the software position control to move to the bead and trap it. There is very limited motion from the softwarecontroller piezo actuators, so you have to be pretty close to the trap (marked with a cross in the camera viewer).
4.3 Equipartition Method
- In the ‘Recording’ tab of the software, choose to record ‘X-dff’, ‘Y-diff’ and ‘Sum’. Select ‘Single-Shot Mode’ and decide on how many samples and how much time you can record data for. Remember that you can only log 48,000 measurements/second and that you are interested in the average behaviour of the bead over a relatively long period of time, like seconds.
- Take data with a few different maximum amounts of time, up to maybe about 10 or 20 seconds.
- Increase the current up to 250 mA and repeat your measurements.
- Decrease the current and take several more measurements between 200 and 80 mA. Be sure to do the lowest current last since the bead may well escape such a shallow potential.
- Note that you have been measuring the ‘Sum’ channel in order to normalize all of your measurements.
4.4 Stokes Drag Method
The student has some leeway in how this experiment is performed. The bead is dragged by the trap as it oscillates back and forth in either the x or y direction. According to Equation (3), both the position and velocity of the bead must be known to determine the trap stiffness, which can be accomplished using many measurements of triangle waves operated at different frequencies, or one sine wave, since a sine wave smoothly transitions through a range of velocities.
- Start with a laser power between 200 and 250 mA.
- In the ‘Oscillations’ menu, select your desired wave, starting position, and amplitude. Try to use a starting position near to where your bead is trapped, an amplitude of 1-2
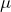 m, and a frequency of 1-2 Hz.
m, and a frequency of 1-2 Hz. - Since this technique can only measure one axis at a time, you can set the recording only for the x or y values that you will need, along with the ‘Sum’ channel.
- If you start the oscillations, you should see the trap and bead moving in your chosen direction. It may be hard to tell that it’s moving since there likely aren’t any points of reference. Go ahead and record data for a few oscillations. You should see the desired waveform in the strain gauge measurement and a changing signal in the QPD measurement.
- Start by lowering the laser current and taking another data set, then lowering again and taking another data set. Hopefully you can get as low as you did in the equipartition method. Too low a current and you may lose your bead, so proceed with caution.
- If you were using the sine wave, then the data taking is done and the more complicated analysis would begin.
- If you are using the triangle wave, then change the frequency and repeat all of those measurements. You will want about 5 frequency measurements per laser current.
- By differentiating the X-strain signal and plotting the deflection of the bead vs this velocity, you will be able to fit a line to your experimental data and measure the stiffness of the trap.
4.5 Stuck Bead Calibration
- Turn the laser current down to zero and open the laser enclosure. Move the stage away from the objective in the z-axis until the slide cover is in focus. You may already see the dots that are the stuck beads. If not, then move the sample so that the tape edge is in view. There are usually lots of stuck beads at the edges – just don’t use a bead that is in the tape or very close to some other beads.
- Do your best to center the trap on the stuck bead, then activate the triangle wave oscillations at a frequency of about 0.1 Hz and an amplitude of 1
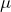 m. Position the trap so that you get a maximum change in QPD signal in the axis of oscillation. Use a larger amplitude if that helps.
m. Position the trap so that you get a maximum change in QPD signal in the axis of oscillation. Use a larger amplitude if that helps. - Set the recorder so that you save a couple sweeps of the bead. The measurement frequency can be low since the stage is moving so slowly.
- Plot the QPD response vs strain-gauge signal and fit the linear region. Use that fit and the strain-gauge calibration data to calibrate the QPD response in terms of
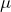 m.
m. - Repeat for the other axis. You now have a calibration factor so that you can turn the QPD measurements into actual distances as read by the strain gauge.
