Optical Tweezers
Apparatus
A schematic of the apparatus is shown in Figure 3. The whole thing is in an aluminium enclosure to protect you from the high-power laser that would blind you before you could blink.
3.1 SAFETY
The safest way to operate the laser is to:
- Make sure that the TEC is on and set to some resistance value near 10 k
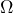 .
. - Make sure that the door to the laser enclosure is closed and the screw turned so that the door stays closed.
- Turn on the laser controller if it isn’t already on.
- Increase the current to your desired value, no higher than 300 mA.
- Be sure to turn the current down to 0 mA before you open the laser enclosure.
3.2 Laser
The path of the laser beam is shown in Figure 3. The laser light is generated by the fiber-coupled laser diode at the back of the enclosure where it is mounted along with control electronics and heat-sinks. The source is a Fiber Bragg Grating stabilized single mode laser diode (Thorlabs PL980P330J; 980 nm, 330 mW). It is directly coupled to the yellow fiber optic visible in the back corner of the enclosure to be coupled into the apparatus.
The laser output is set with two controllers: The Thorlabs LDC 210C and TED 200C. The former provides control over the drive current and other electrical inputs to the laser diode itself, and the latter controls the thermoelectric cooler (TEC) that controls the temperature of the laser diode. The output and stability of a laser diode is quite sensitive to the diode temperature (which can change with both drive parameters and environmental factors), and so good thermal control is necessary for laser stability.
The output of the fiber is passed through a beam-expander in order to fill the area of the objective further downstream. The beam is reflected off the dichroic and regular mirror, to the aperture of a 100X oil immersion objective lens (0.23mm working distance, NA 1.25), and focused within the sample plane. In this technique, the lens and the sample slide are both immersed in an oil with a high refractive index, increasing the resolving power of the microscope and the numerical aperture of the specially designed lens, thereby creating a well defined (near diffraction-limited) and tightly focused beam waist.
On the other side of the sample, the laser light is collected in the condenser lens, reflected off of another dichroic mirror and onto the QPD for imaging.
3.3 White Light
The sample is illuminated by an LED source from above the dichroic filter in the vertical section of Figure 3. The light passes through the condenser and is focused on the sample plane by the condenser lens. The visible light is collected from the sample area by the objective lens and directed back through the dichroic filter (the beam expander output filter) and passes through onto the camera section. The camera is located behind a short-pass filter (not shown on schematic) to protect the camera from any intense back-migrating laser light and the sample plane is imaged by the camera lens onto the camera plane.
Figure 4: Schematic of the quadrant pole detector (QPD). The X-diff, Y-diff, and Sum values are determined from the output current of the photodiode quadrants. The red circle represents the laser beam.
3.4 Stage Movement
3.4.1 Manually
The sample itself is held on the XYZ controlled stage. The stage has micrometer adjustment of the X,Y and Z positions. Students should use these stage controls to get a bead in the field of view of the camera. Since these controls are inside the laser enclosure, they cannot be operated while the laser is on.
3.4.2 Electronically
Piezo-actuators are used to achieve small, accurate movements and to allow computer control of the stage. These actuators provide a nominal 20 ![]() m distance of travel for each axis. Due to the effects of hysteresis originating both in the piezoelectric materials themselves as well as at the mechanics of the actuators, the relationship between the voltage applied to the piezo-actuators is not strictly linear even within the elastic range of the materials. A separate measurement must be made in order to accurately know the position of the stage.
m distance of travel for each axis. Due to the effects of hysteresis originating both in the piezoelectric materials themselves as well as at the mechanics of the actuators, the relationship between the voltage applied to the piezo-actuators is not strictly linear even within the elastic range of the materials. A separate measurement must be made in order to accurately know the position of the stage.
3.5 Detectors
3.5.1 Quadrant Pole Detector
The quadrant pole detector (QPD) is shown in Figure 4. As the bead moves to a different position in the trap, the laser beam will be deflected by refraction and the position of the image of the beam on the QPD plane will move. If the beam image moves up, the upper set of photodiodes will be see a larger fraction of the total beam power while the lower set of photodiodes will see a lower fraction of the total beam power. If the beam image moves left, the left set of photodiodes will be see a larger fraction of the total beam power while the right set of photodiodes will see a lower fraction of the total beam power. By subtracting the signals of the two lower detectors from the two upper detectors as well as the two left detectors from the two right detectors and normalizing these values, the center of the (symmetric) beam image relative to the center of the detector can be measured giving an X and Y position signal. The output of the QPD is calibrated using a stuck bead of known diameter.
The measurement channels are separated into ‘X-diff’, ‘Y-diff’, and ‘Sum’. The ‘Sum’ channel is necessary to normalize the measurements and account for light lost due to scattering or changes in the overall power of the laser.
3.5.2 Strain Gauges
The piezo-actuators can’t be relied on for repeatably accurate amounts of distance per applied voltage, and so the actuators of the X and Y axis are fitted with built-in strain sensors which are able to measure the strain of the actuators (and hence the stage deflection) without hysteresis. It is this strain amount that will be used to calibrate the output of the QPD using the stuck bead.
3.6 Data Acquisition
In general, communication via USB is slow compared to the timescales of interest in these experiments. The outputs of QPD and the strain gauges are fed through a relatively high bandwidth data acquisition (DAQ) card which monitors the raw output of the QPD amplifiers and strain sensor bridge circuits to provide fast measurements proportional to the QPD difference and sum signals and strain gauge position readings. The DAQ card is limited by a total throughput of 48,000 Samples/sec and so as the number of signals monitored concurrently increases, the sample rate per channel decreases proportionally (max sample rate = 48,000 Samples/#channels/s). It is therefore recommended that only the variables of interest are recorded during each experimental run.
This experiment is controlled with a program written in LabView. Instead of written lines of code like Python, LabView is a graphical programming language where data is passed by ‘wires’ that connect virtual representations of lab equipment. LabView programs have a ‘front panel’, which acts as the user interface. Controls and indicators on the front panel allow the user to operate the experiment electronically but in a very intuitive way. The program has two tabs that you will need to access.
3.6.1 Initialization
The initialization routine of the stage controllers provides you with two [![]() m; V ] points on the ends of the linear portion of the stage movement for each axis. You can use these to convert the strain gauges signal in your output file directly to a position signal in
m; V ] points on the ends of the linear portion of the stage movement for each axis. You can use these to convert the strain gauges signal in your output file directly to a position signal in ![]() m. This initialization routine runs each time the LabView program is started. If for some reason the program crashes or the PC loses power, you will need to record the new calibration data to use for subsequent data sets.
m. This initialization routine runs each time the LabView program is started. If for some reason the program crashes or the PC loses power, you will need to record the new calibration data to use for subsequent data sets.
3.6.2 Quick Move
The ‘Quick Move’ tab has controls for the piezo actuators. The user can move the stage by click-dragging in the position square with a range of 20 ![]() m. The z-axis is controlled by scrolling the mouse wheel. The position of the trap is stated near the position square – be aware of that starting position when you start your oscillations. Controls for the oscillations are also in this tab. The user can oscillate the trap in the x OR y direction. The ‘Quick Move’ controls for the direction of oscillation are disabled, but the other directions are enabled.
m. The z-axis is controlled by scrolling the mouse wheel. The position of the trap is stated near the position square – be aware of that starting position when you start your oscillations. Controls for the oscillations are also in this tab. The user can oscillate the trap in the x OR y direction. The ‘Quick Move’ controls for the direction of oscillation are disabled, but the other directions are enabled.

