Chapter 3: Exercise Metabolism and Bioenergetics
Protein Metabolism
Protein Metabolism
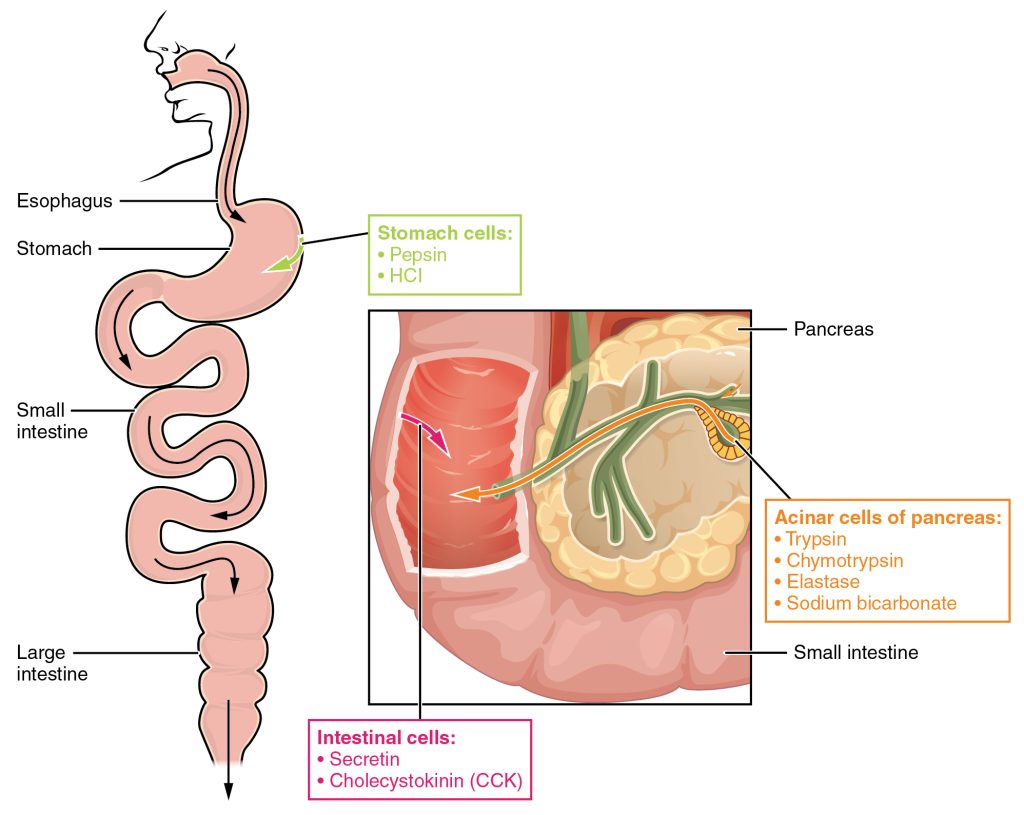
The digestion of proteins begins in the stomach. When protein-rich foods enter the stomach, they are greeted by a mixture of the enzyme pepsin and hydrochloric acid (HCl; 0.5 percent). The latter produces an environmental pH of 1.5–3.5 that denatures proteins within food. Pepsin cuts proteins into smaller polypeptides and their constituent amino acids. When the food-gastric juice mixture (chyme) enters the small intestine, the pancreas releases sodium bicarbonate to neutralize the HCl. This helps to protect the lining of the intestine. The small intestine also releases digestive hormones, including secretin and CCK, which stimulate digestive processes to break down the proteins further. Secretin also stimulates the pancreas to release sodium bicarbonate. The pancreas releases most of the digestive enzymes, including the proteases trypsin, chymotrypsin, and carboxypeptidase, which aid protein digestion. Together, all of these enzymes break complex proteins into smaller individual amino acids (Figure 3.21), which are then transported across the intestinal mucosa to be used to create new proteins, or to be converted into fats or acetyl CoA and used in the Krebs cycle.
In order to avoid breaking down the proteins that make up the pancreas and small intestine, pancreatic enzymes are released as inactive proenzymes that are only activated in the small intestine. In the pancreas, vesicles store trypsin, chymotrypsin, and procarboxypeptidase as trypsinogen, chymotrypsinogen, and carboxypeptidase, respectively. Once released into the small intestine, an enzyme found in the wall of the small intestine, called enterokinase, binds to trypsinogen and converts it into its active form, trypsin. Trypsin then binds to chymotrypsinogen to convert it into the active chymotrypsin. Trypsin and chymotrypsin break down large proteins into smaller peptides while carboxypeptidase cleaves individual amino acids, a process called proteolysis. These smaller peptides are catabolized into their constituent amino acids, which are transported across the apical surface of the intestinal mucosa in a process that is mediated by sodium-amino acid co-transporters. These transporters bind sodium and then bind the amino acid to transport it across the membrane. At the basal surface of the mucosal cells, the sodium and amino acid are released. The sodium can be reused in the transporter, whereas the amino acids are transferred into the bloodstream to be transported to the liver and cells throughout the body for protein synthesis.
Freely available amino acids are used to create proteins. If amino acids exist in excess, the body has no capacity or mechanism for their storage; thus, they are converted into glucose or ketones, or they are decomposed. Amino acid decomposition results in hydrocarbons and nitrogenous waste, which includes ammonia which is converted to urea in the liver via the urea cycle. This process produces a keto group that may be used in the Krebs cycle and hence is used as a source of energy.
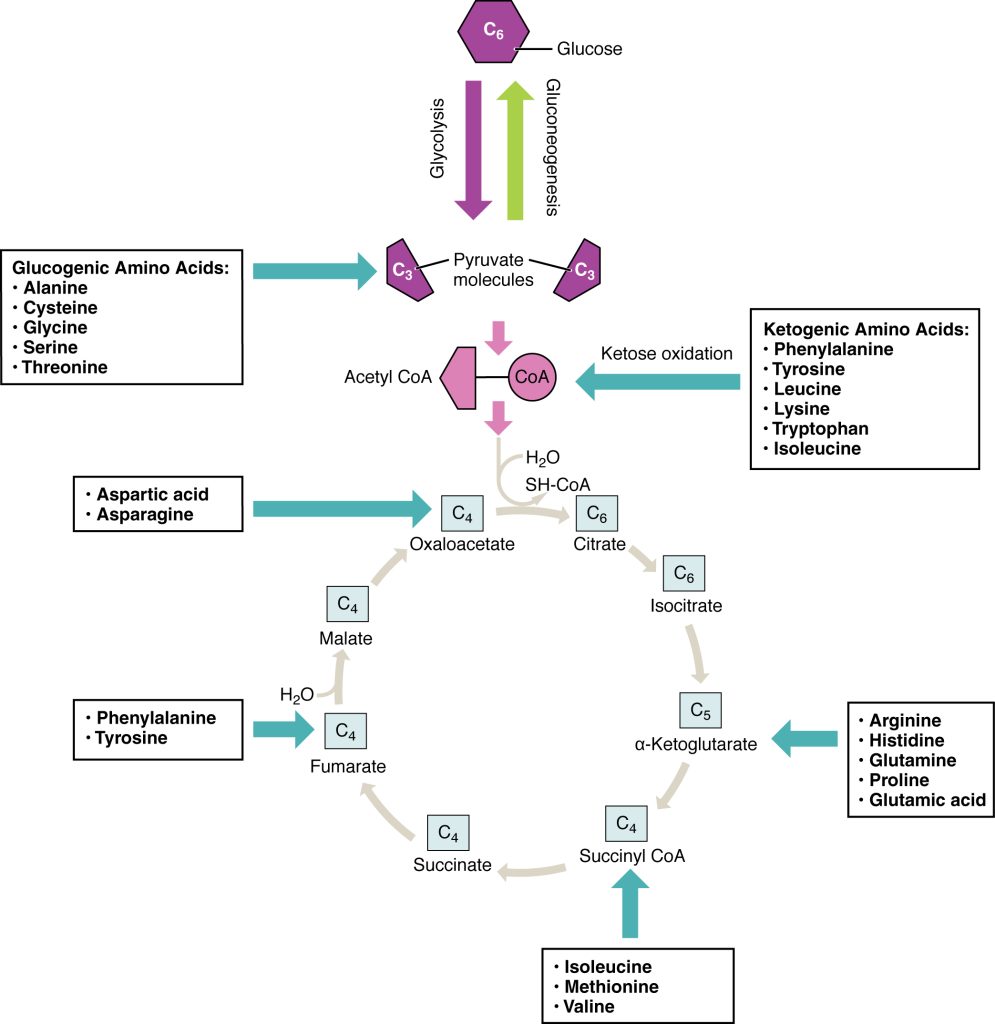
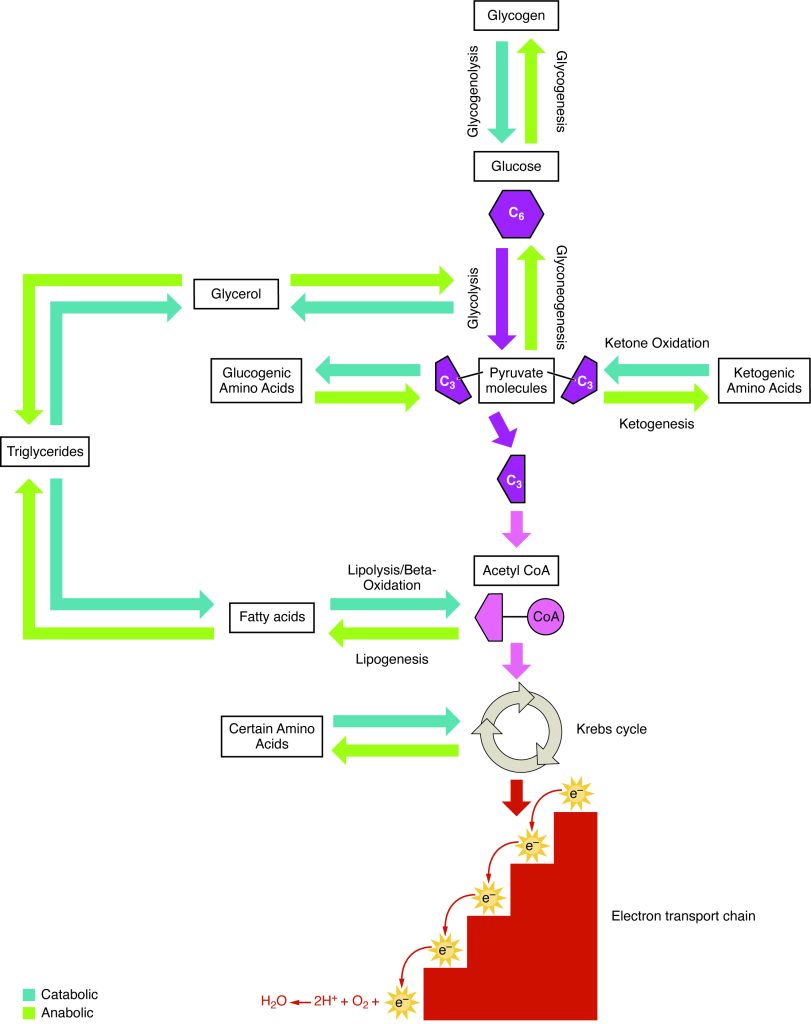
Amino acids can also be used as a source of energy, especially in times of starvation. Because the processing of amino acids results in the creation of metabolic intermediates, including pyruvate, acetyl CoA, acetoacyl CoA, oxaloacetate, and α-ketoglutarate, amino acids can serve as a source of energy production through the Krebs cycle (Figure 3.22). Figure 3.23 summarizes the pathways of catabolism and anabolism for carbohydrates, lipids, and proteins.
Chapter Review
Digestion of proteins begins in the stomach, where HCl and pepsin begin the process of breaking down proteins into their constituent amino acids. As the chyme enters the small intestine, it mixes with bicarbonate and digestive enzymes. The bicarbonate neutralizes the acidic HCl, and the digestive enzymes break down the proteins into smaller peptides and amino acids. Digestive hormones secretin and CCK are released from the small intestine to aid in digestive processes, and digestive proenzymes are released from the pancreas (trypsinogen and chymotrypsinogen). Enterokinase, an enzyme located in the wall of the small intestine, activates trypsin, which in turn activates chymotrypsin. These enzymes liberate the individual amino acids that are then transported via sodium-amino acid transporters across the intestinal wall into the cell. The amino acids are then transported into the bloodstream for dispersal to the liver and cells throughout the body to be used to create new proteins. When in excess, the amino acids are processed and stored as glucose or ketones. The nitrogen waste that is liberated in this process is converted to urea in the urea acid cycle and eliminated in the urine. In times of starvation, amino acids can be used as an energy source and processed through the Krebs cycle.
Glossary
- carboxypeptidase
- pancreatic enzyme that digests protein
- chymotrypsin
- pancreatic enzyme that digests protein
- chymotrypsinogen
- proenzyme that is activated by trypsin into chymotrypsin
- enterokinase
- enzyme located in the wall of the small intestine that activates trypsin
- inactive proenzymes
- forms in which proteases are stored and released to prevent the inappropriate digestion of the native proteins of the stomach, pancreas, and small intestine
- pepsin
- enzyme that begins to break down proteins in the stomach
- procarboxypeptidase
- proenzyme that is activated by trypsin into carboxypeptidase
- proteolysis
- process of breaking proteins into smaller peptides
- secretin
- hormone released in the small intestine to aid in digestion
- sodium bicarbonate
- anion released into the small intestine to neutralize the pH of the food from the stomach
- trypsin
- pancreatic enzyme that activates chymotrypsin and digests protein
- trypsinogen
- proenzyme form of trypsin