11 2.2 Stroke and Loss of Blood Flow as an Acute Injury to the Brain
The lack of blood flow to an area of the brain known as Ischemia. This is dangerous to the brain as a lack of circulating blood deprives the neurons of oxygen and nourishment. This ischemia that underlies stroke can occur in 2 different ways, hemorrhage: release of blood from blood vessels after the vessel rupture causes damage by cutting off connecting pathways, resulting in local or generalized pressure injury as well as impaired blood flow to the brain, and blocking a blood vessel causing a lack of blood flow to that region of the brain as shown in Figure 1.
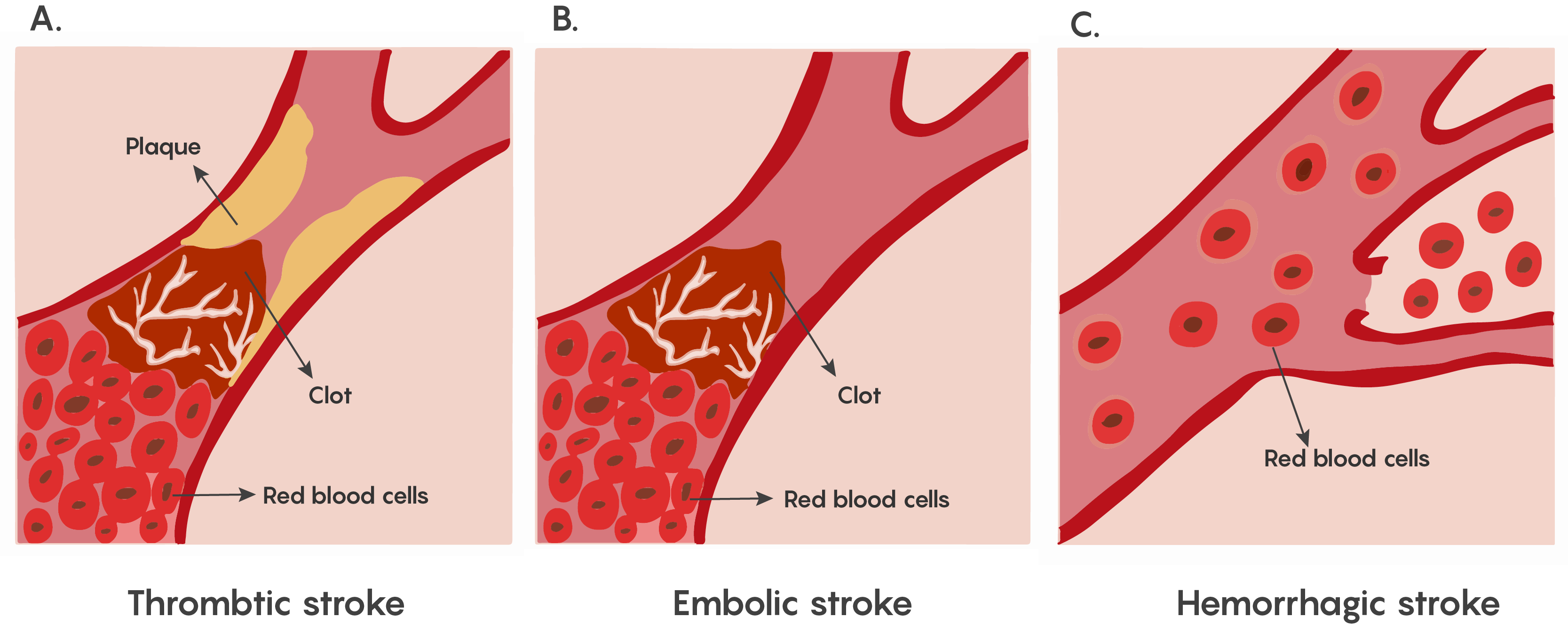
Depending on the area of the brain where this lack of blood flow has occurred, functions that the specific area of the brain normally performs such as movement, sensation, or emotions, speech etc. may be affected and the loss of function varies with location and extent of damage.
Most forms of ischemia in stroke involve the thrombotic or embolic types of blockage, and a much smaller percentage involve hemorrhage. Although both are serious, all strokes have a therapeutic window that allows for neurons that are undergoing ischemia to be rescued.
Cellular Mechanisms underlying ischemia induced cell death
The brain requires a continuous supply of O2 and glucose for neurons to function. If the cerebral blood flow is interrupted, then individual neuronal metabolism can be affected within 30 seconds and will completely stop within 2 minutes of deprivation. If left unchecked, neuronal cell death occurs in 5 minutes. During this time period these neurons will no longer be able to maintain their resting membrane potentials and when they die, will release K+ ions causing other nearby cells to depolarize. In addition, as Na+/K+ATP dependent pumps will no longer have ATP production to drive their activity, the neurons will also start to depolarize and fire action potentials inappropriately. This leads to the glutamate excitotoxicity theory proposed by John Olney and may be the basis of why pyramidal neurons that are found in the hippocampus and Purkinje cells in the cerebellum that rely on glutamate neurotransmission are particularly vulnerable and die following ischemia.
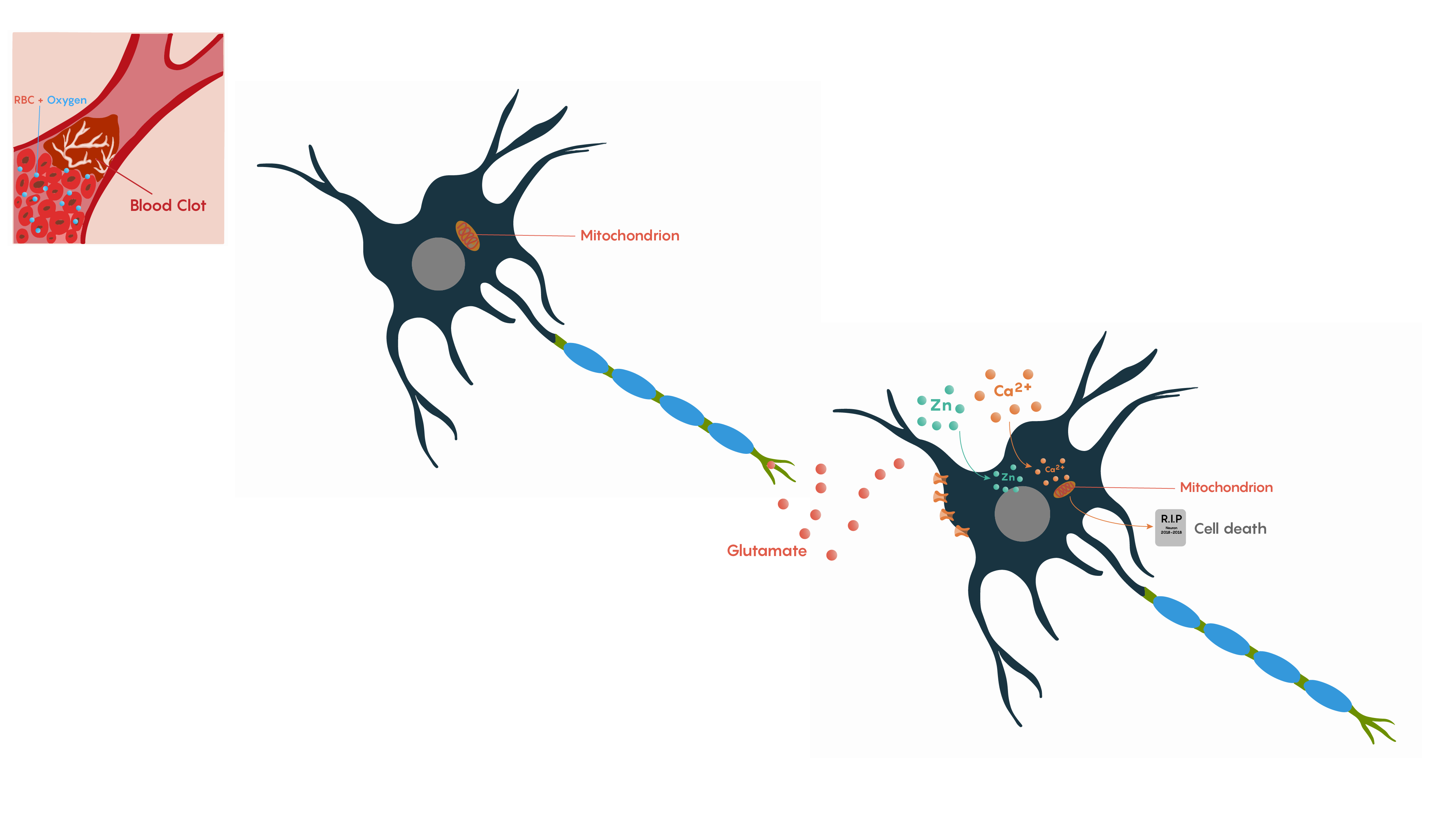
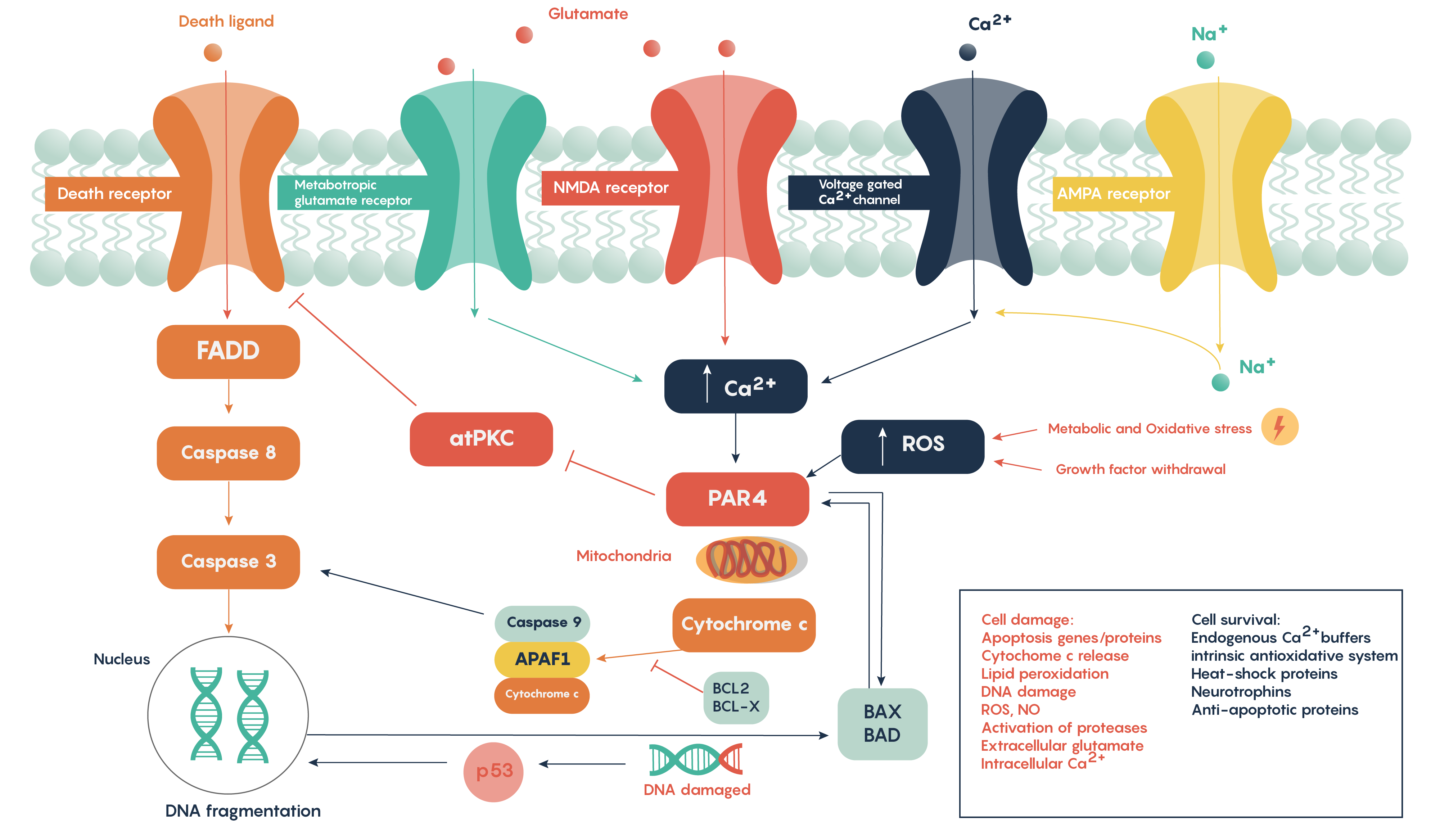
Following ischemia to a region of neurons, the increased release of glutamate and its decreased uptake without ATP production (Figure 2.) produces an excess of glutamate which impacts downstream neurons. Glutamate will impact a number of glutamate specific receptors such as the AMPA, NMDA and metabotropic type receptors which in turn will cause activation of voltage gated Ca2+ channels, all of which result in the increase in intracellular Ca2+ levels. In turn, this will activate the protease activated receptor PAR4 which will activate the pro-apoptotic proteins BAX/BAD that will then form a complex with the mitochondrial factor cytochrome c which is released from the mitochondrial matrix as the intracellular ATP stores fail. This complex in turn will then activate Caspase 9 and the downstream and ultimate factor Caspase 3 which is involved in the apoptotic DNA fragmentation and eventual death of the neuron. As this is a programmable/apoptotic form of cell death, there are a number of therapeutic interventions that may prevent this stroke and ischemia induced form of cell death.
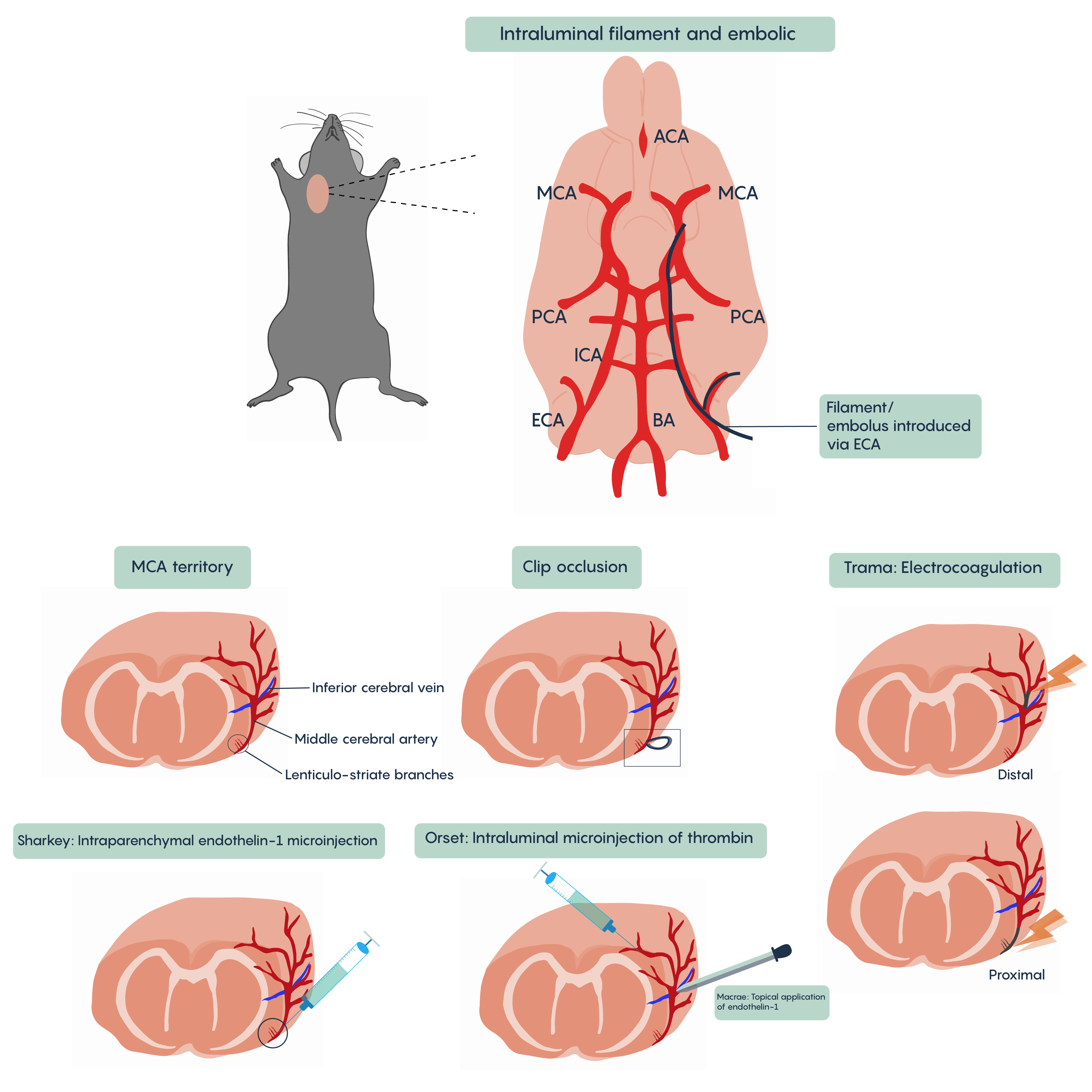
A number of different animal models of stroke have been developed. Most involve interruptions in blood flow such as the intraparenchymal (i.e. directly into the brain tissue) injection of drugs such as endothelin-1 (Et-1) which induces vasospasms of the blood vessels creating ischemia, of the introduction of coagulants such as thrombin injection that will block blood vessels (Figure 4. Animal models of stroken). Most methods will involve blockade of normal blood flow to an area of the brain to produce ischemia whether through occlusion of a blood vessel such as the middle cerebral artery (MCA) or the chemical methods described here. These animal models have proved invaluable in understanding the role of different factors such as cytochrome c or BAD/BAX in excitotoxicity and hold the promise of developing therapeutic interventions.

