3.1.4 – SN1 vs SN2
Comparison Between SN1 and SN2 Reactions
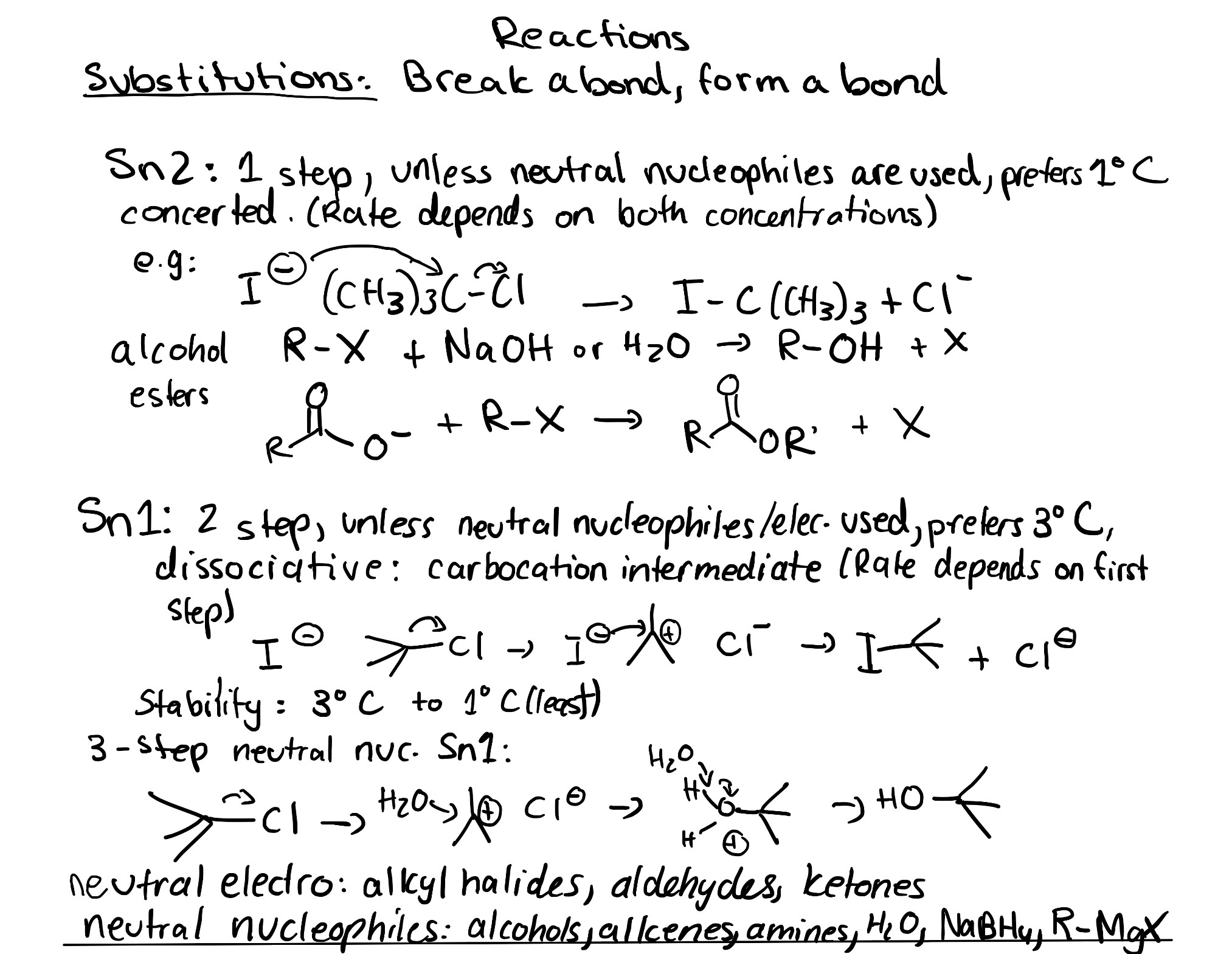
This past chapter has taught us the basic concepts about SN1 and SN2 reactions. There are some similarities between the two reactions. For example, both SN1 and SN2 are substitution reactions where an electrophile is subject to nucleophilic attack. The electrophile contains a carbon centre with a positive or partial positive charge, such as a carbocation or an alkyl halide. Another similarity between SN1 and SN2 reactions is the nucleophile, which has a lone pair of electrons to donate to the electrophilic carbon atom. The nucleophile could be a halide, a hydroxide or alkoxide, water or an alcohol, among others. Another similarity between SN1 and SN2 is the leaving group, which must leave to allow for the nucleophile and electrophile to react. This will result in a functional group “switch” or substitution.
Despite those similarities, there are many differences between the two. Below is a table summarizing the key differences. Try making your own table or summary to help with your understanding.
| SN1 | SN2 | |
| Rate Law | Rate = k[Alkyl Halide] | Rate = k[Nucleophile]x[Alkyl Halide] |
| Mechanism | Multiple steps; dissociative | One step; concerted |
| Presence of an Intermediate? | Yes (Carbocation) | No |
| Reaction Diagram | 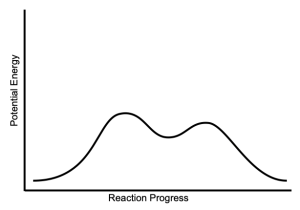 |
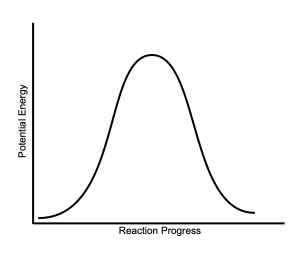 |
| Electrophilic Substrate | tertiary 3˚ > secondary 2˚ > primary 1˚ and methyl | primary 1˚ and methyl > secondary 2˚ > tertiary 3˚ |
| Reason for Preference | Electron density stabilization | Steric effect |
| Alternative Reactions |
|
|
| Nucleophile | Typically involves neutral nucleophiles such as water | Typically involves anionic nucleophiles like hydroxide |
The best way to determine the order of reactivity based on electrophilic substrate is whether the carbon bonded to the halogen is more substituted, or less substituted. If a carbon atom is said to be more substituted, it is bonded to less hydrogen atoms, and more substituents, such as other carbon chains. SN1 reactions favour more substituted carbons due to electron density stabilization. If a carbon atom is said to be less substituted, it is bonded to more hydrogen atoms, and less substituents. SN2 reactions favour less substituted carbons due to sterics.
The Choice of Reaction Pathway: SN1 or SN2?
The reaction pathway predominantly depends on the nature of the substrate (primary, secondary, or tertiary), as shown in Figure 3.1.4.a.
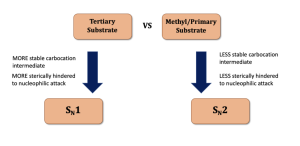
- Primary and methyl substrates predominantly undergo SN2 reactions.
- Tertiary substrates will undergo an SN1 process.
- The reaction of secondary substrates mainly relies on the conditions applied (which will be further discussed in year 2 organic chemistry).
(The full solution to this problem can be found in Chapter 5.2)
The following video includes a worked example from a previous CHEM 1AA3 test or exam that students struggled with. Try solving it on your own before looking at the solution.
Key terms in this chapter:
| Key term | Definition |
| Dissociative | A mechanism where bonds are broken and formed in multiple steps. This is typically seen when a neutral nucleophile attacks a sterically hindered electrophile, with the first step being the loss of a leaving group followed by a nucleophilic attack (SN1 reaction). |
| Concerted | A mechanism in which bonds are broken and formed simultaneously, occurring all in one step. This is typically seen when an anionic nucleophile attacks an electrophile that is not sterically hindered (such as in SN2 reactions). |
| More substituted | Refers to a carbon atom that is bonded to less hydrogen atoms and more substituents, such as other carbon atoms. SN1 reactions favour more substituted carbons. |
| Less substituted | Refers to a carbon atom that is bonded to more hydrogen atoms and less substituents. SN2 reactions favour less substituted carbons. |
Study Notes
Any feedback or comments on this chapter? You may either email chemoer@mcmaster.ca, access this MS Form, or provide a comment in the feedback box below.
A mechanism where bonds are broken and formed in multiple steps. This is typically seen when a neutral nucleophile attacks a sterically hindered electrophile, with the first step being the loss of a leaving group followed by a nucleophilic attack (SN1 reaction).
A mechanism in which bonds are broken and formed simultaneously, occurring all in one step. This is typically seen when an anionic nucleophile attacks an electrophile that is not sterically hindered (such as in SN2 reactions).
Refers to a carbon atom that is bonded to less hydrogen atoms and more substituents, such as other carbon atoms. SN1 reactions favour more substituted carbons.
Refers to a carbon atom that is bonded to more hydrogen atoms and less substituents. SN2 reactions favour less substituted carbons.

