5.2 – Solutions for Chapter 3 – Reactivity
Chapter 3.1.1 – Introduction to Nucleophiles, Electrophiles and Curved Arrows
1. Which of the following are electrophiles?
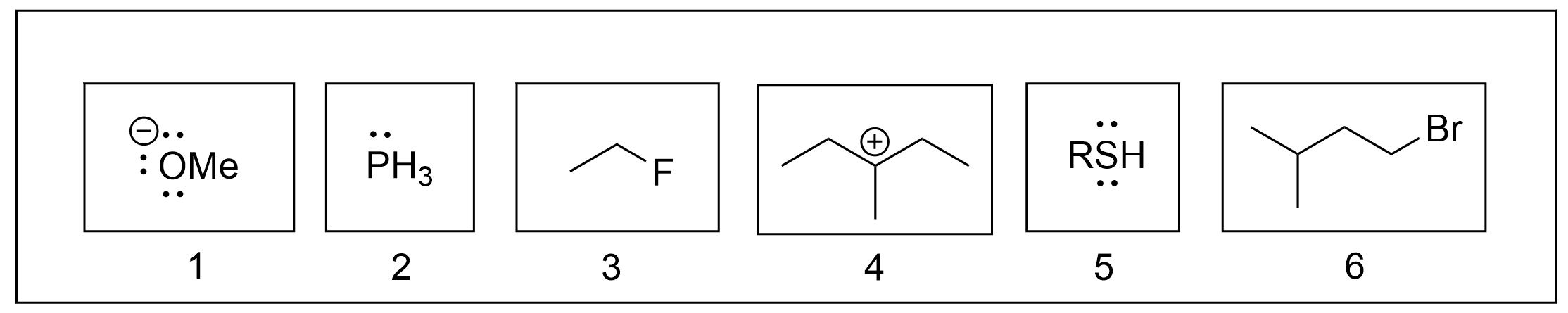
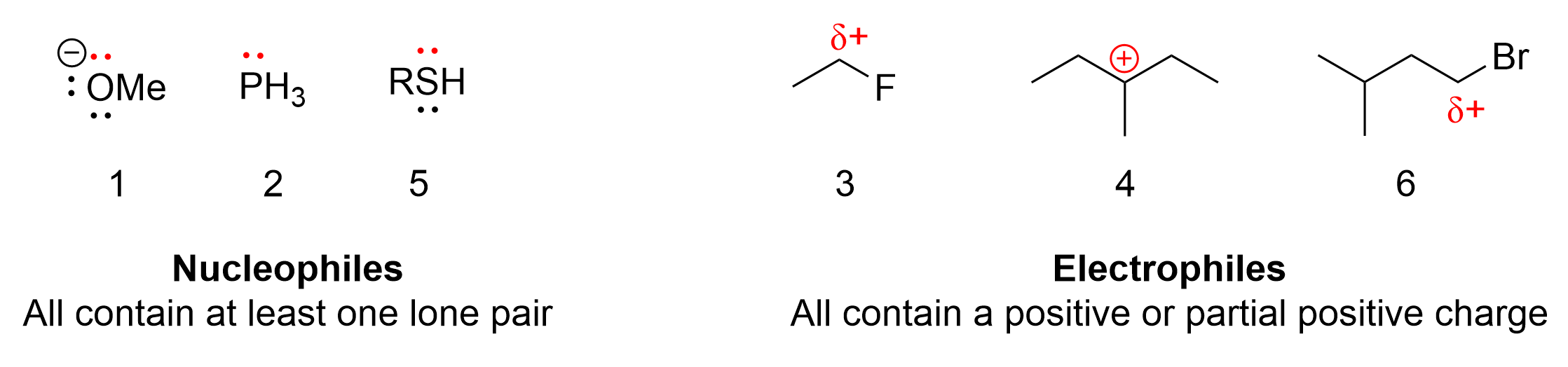
The correct answers to this question are compounds 3, 4, and 6. The easiest way to identify electrophiles is to look for either a positive charge or a partial positive charge on a molecule. Positive charges will be clear (as seen with a + symbol), whereas partially positive carbons will be bonded to a highly electronegative atom, such as halides (Cl, Br, F, I) or oxygen. Molecules 3 and 6 both contain a halide, a highly electronegative group. Therefore, the carbon it is bonded to is partially positive, making it an electrophile. Molecule 4 contains a carbon with an incomplete octet, as seen by the positive charge, making it an electrophile.
Nucleophiles, in contrast, can be identified by having at least one lone pair, typically found on a heteroatom such as oxygen, phosphorus, or sulfur. This is seen on molecules 1, 2 and 5. There are no partially or fully positive charges on these molecules, as they mainly consist of lone pairs. These lone pairs can be used to form a new bond with another molecule, making them nucleophiles.
2. Which of the following arrow-pushing reaction diagrams is correct?
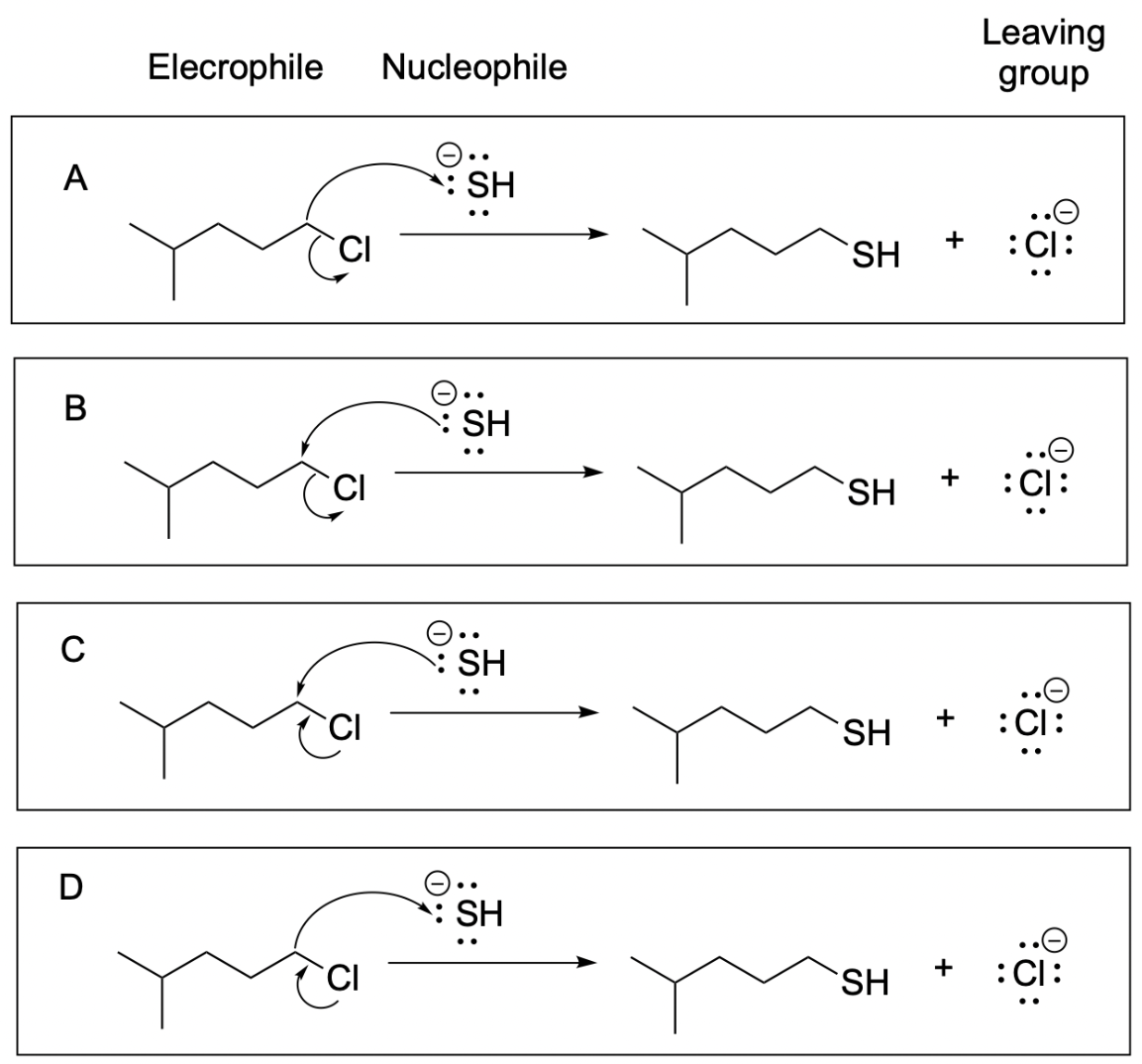
The correct answer to this question is Option B.
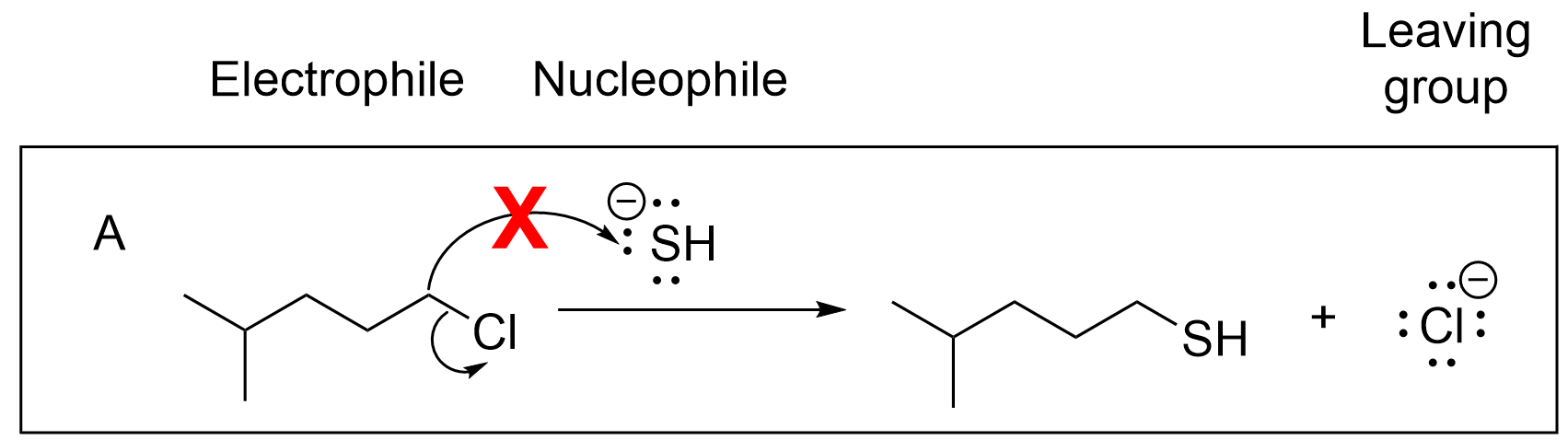
Option A is incorrect due to the direction of the first arrow. Recall that arrows must start at the source of electron (the nucleophile) and point towards the electron-deficient area (the electrophile). This arrow is pointing in the opposite direction; there are no lone pairs on the carbon to move, and there is also no need for sulfur, which has a full octet, to gain more electrons. Thus, this answer is wrong.
Option B shows the correct arrow pushing mechanism. The arrow starts at the nucleophile (source of electrons) and points towards the electron-deficient carbon. As this carbon has a full octet, it must lose a bond to make room for the new bond to sulfur. This is seen in the second arrow, pointing from the bond (source of electrons) to the electronegative chlorine, which takes the lone pair with it.
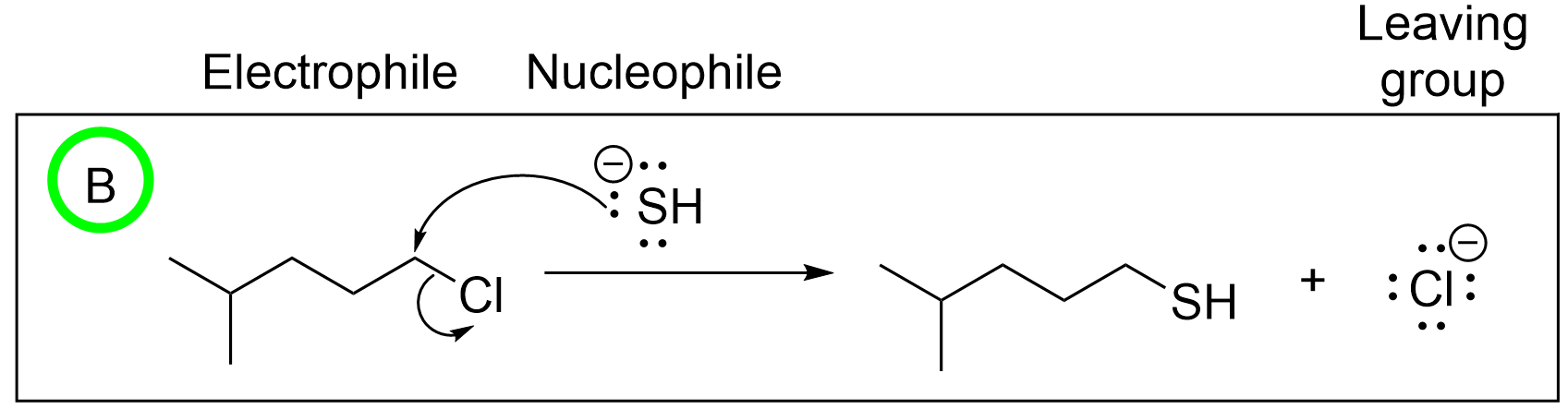
Option C is incorrect due to the second arrow. The nucleophile attacks an electrophilic carbon which already contains a full octet, with two explicit bonds and two more implicit bonds to hydrogen. Thus, the second arrow must break a bond. The arrow must start at the bond and move towards the leaving group, which is Cl in this case. This incorrect arrow makes it seem like a new bond is forming from a lone pair on Cl, which is not possible.
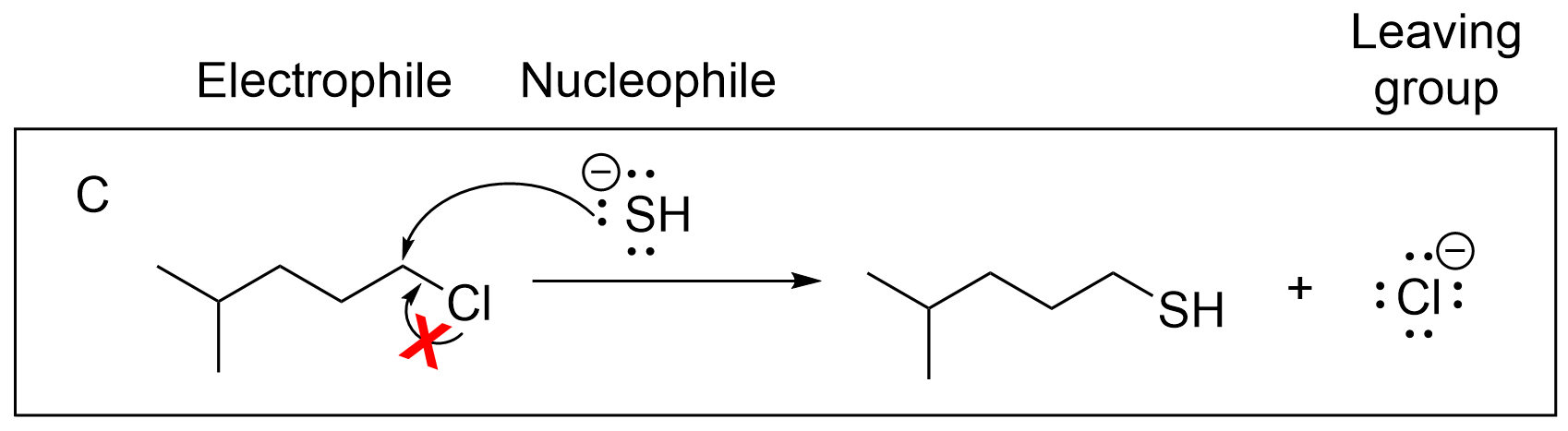
Option D is incorrect as both arrows are pointing in the wrong direction. Recall that arrows must start at the source of electrons (nucleophile) and point towards the electron-deficiency (electrophile). The top arrow is pointing in the opposite direction; there are no lone electrons on the carbon to move, and there is no need for sulfur, which has a full octet, to gain more electrons. The second arrow is also pointing in the opposite direction. As Cl is the leaving group, the C–Cl bond must break. The arrow must start at the bond and move towards the leaving group, which is Cl in this reaction.
3. Which of the following statements regarding organic reactions is FALSE?
A. The nucleophile is the electron donor, and is a Lewis base.
B. Forming a bond in a nucleophilic substitution reaction involves electrons moving from the nucleophile to the electrophile.
C. Nucleophiles and electrophiles must be properly aligned with each other for the reaction to occur.
D. When writing a mechanism, the tail of the double-barbed curly arrow is at the electrophile while the arrowhead is at the nucleophile.
The correct answer is Option D. When drawing mechanisms, the tail always starts at the source of electrons, which would be the nucleophile. The head always points towards the area of electron-deficiency, which would be the electrophile. This is to showcase the movement of electrons. This answer is the opposite of that, and thus, is FALSE and therefore the correct answer to the question.
Options A, B, and C are all true statements, which is not what the question asks for.
Chapter 3.1.2 – SN2 Reaction Mechanism
1. Which of the following is the transitional state of the following reaction? (Ph is the abbreviation of a benzene ring.)
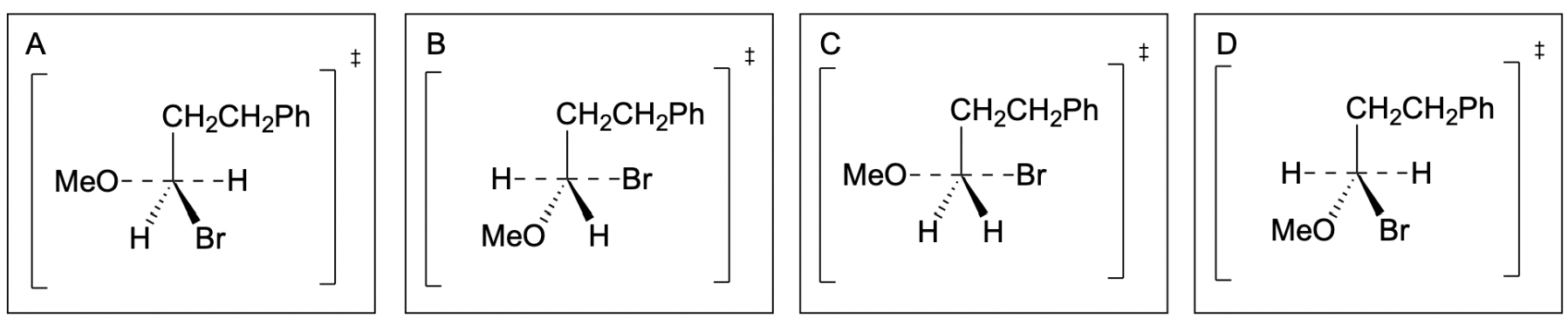
The correct answer to this question is Option C. Recall that in the transition state, the electrophilic carbon consists of 5 total bonds. In the trigonal bipyramidal geometry, the incoming nucleophile and the leaving group are on the same plane as the carbon, appearing on its left and right respectively, and are drawn with partial (dashed) bonds as they are forming and breaking.
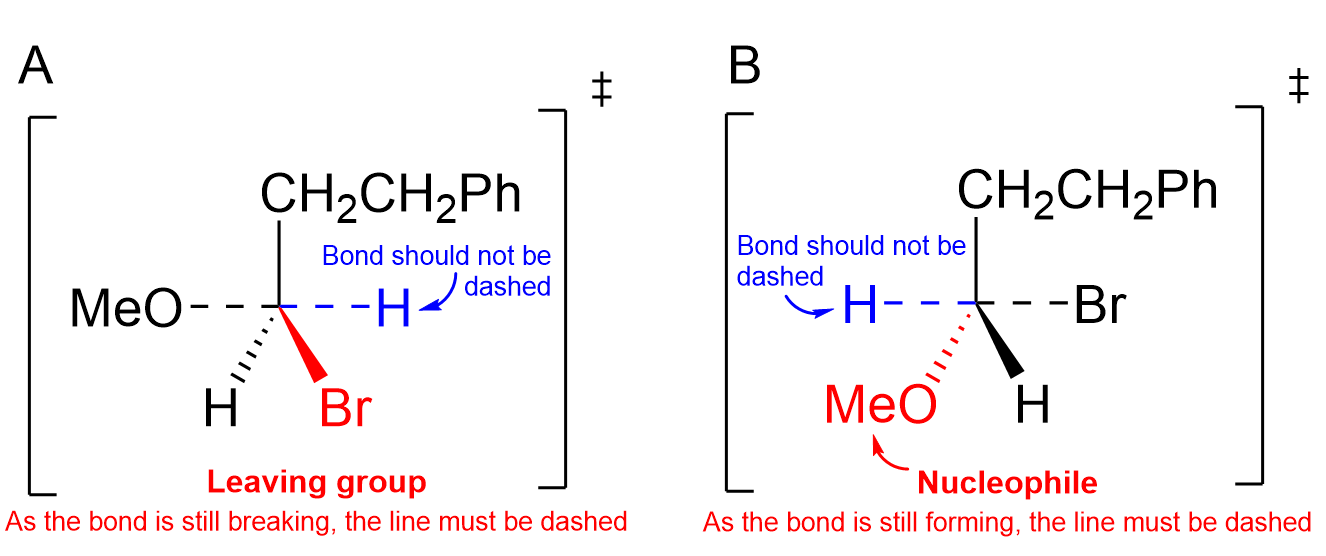
Option A is incorrect as Br, the leaving group in the molecule, is not drawn with a partial bond. As seen in the reaction mechanism, the arrow indicates that the Br is going to leave with a lone pair of electrons. Thus, in the transition state, it should have a partial bond to carbon, depicted with dashed lines, as it is still in the process of leaving. Furthermore, the H bonded to carbon is not leaving or forming a new bond, and thus, should be bonded with a solid line.
Option B is also incorrect as MeO, the nucleophile, is not drawn with a partial bond. As seen in the reaction mechanism, the curved arrow indicates that MeO is attacking the carbon with its lone pair. Thus, in the transition state, it should have a partial bond to carbon, depicted with dashed lines, as it is still in the process of forming the new bond. Furthermore, the H bonded to carbon is not leaving or forming a new bond, and thus, should be bonded with a solid line.
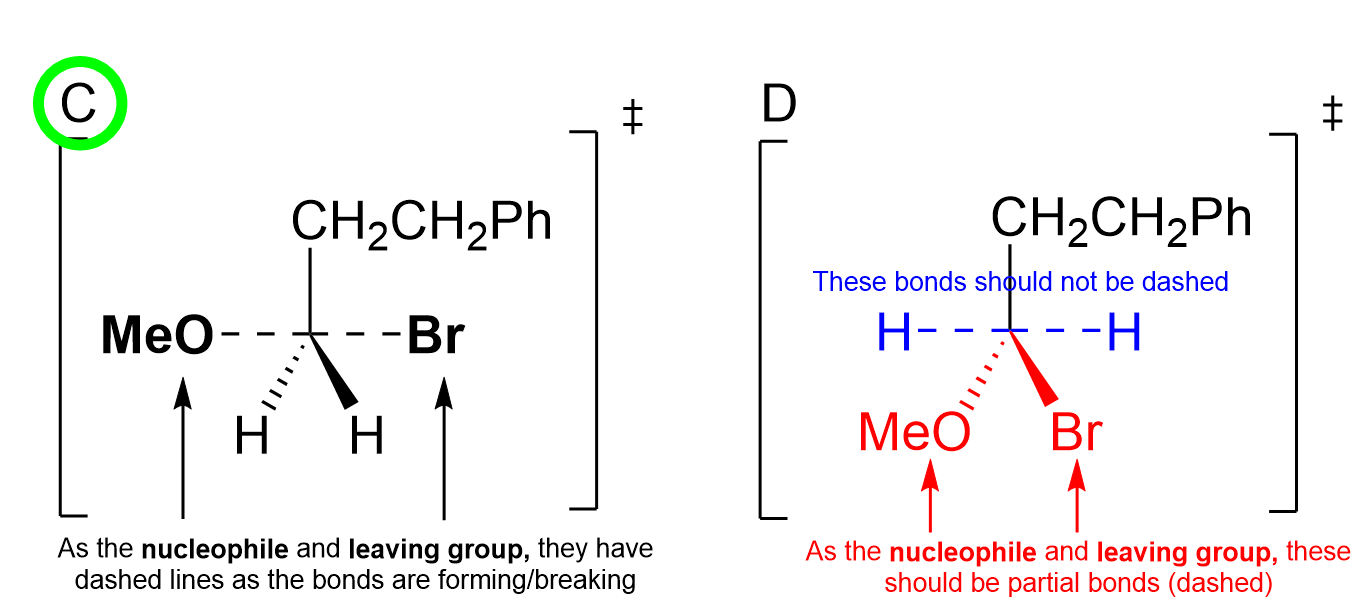
Option C is the correct answer. The nucleophile and leaving group are both drawn with dashed lines to the central carbon, as both bonds are either forming or breaking.
Option D is incorrect, as both the nucleophile (MeO) and the leaving group (Br) are not drawn with partial bonds. The hydrogen atoms should not be drawn with dashed lines, as they are not partial bonds.
Chapter 3.1.3 – SN1 Reaction Mechanisms
1. Which of following is the transition state of the non-rate determining step of the following SN1 reaction?
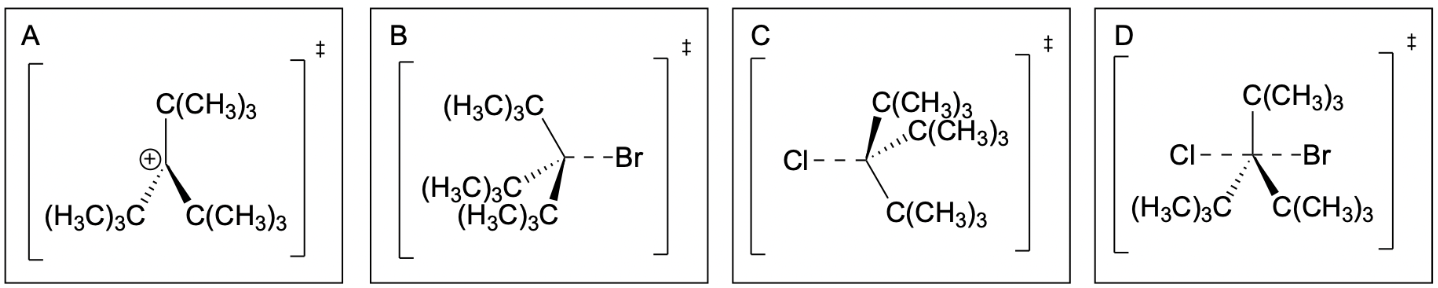
The correct answer to this question is Option B. The first step to answering this question is to understand which step is rate-limiting and which is non-rate-limiting. Recall that the first step of an SN1 reaction is the spontaneous dissociation of the leaving group. This is the rate-limiting step as it occurs slower. Thus, it can be determined that the question is asking for the transition state of the second step, the nucleophilic attack of Br.
Option A is incorrect, as it shows the intermediate of the SN1 reaction, not a transition state. This can be seen as there are no partial bonds and the carbon is positively charged due to the lost leaving group. This is the structure that is obtained after the first step where Cl leaves from the molecule, taking the lone pair of electrons with it.
Option B is correct, as it shows the correct transition state. Since the non-rate-determinant step is the nucleophilic attack of Br, the transition state should show the partially formed bond between the carbon and the Br. Thus, this is the correct answer as it shows the proper dashed line to Br.
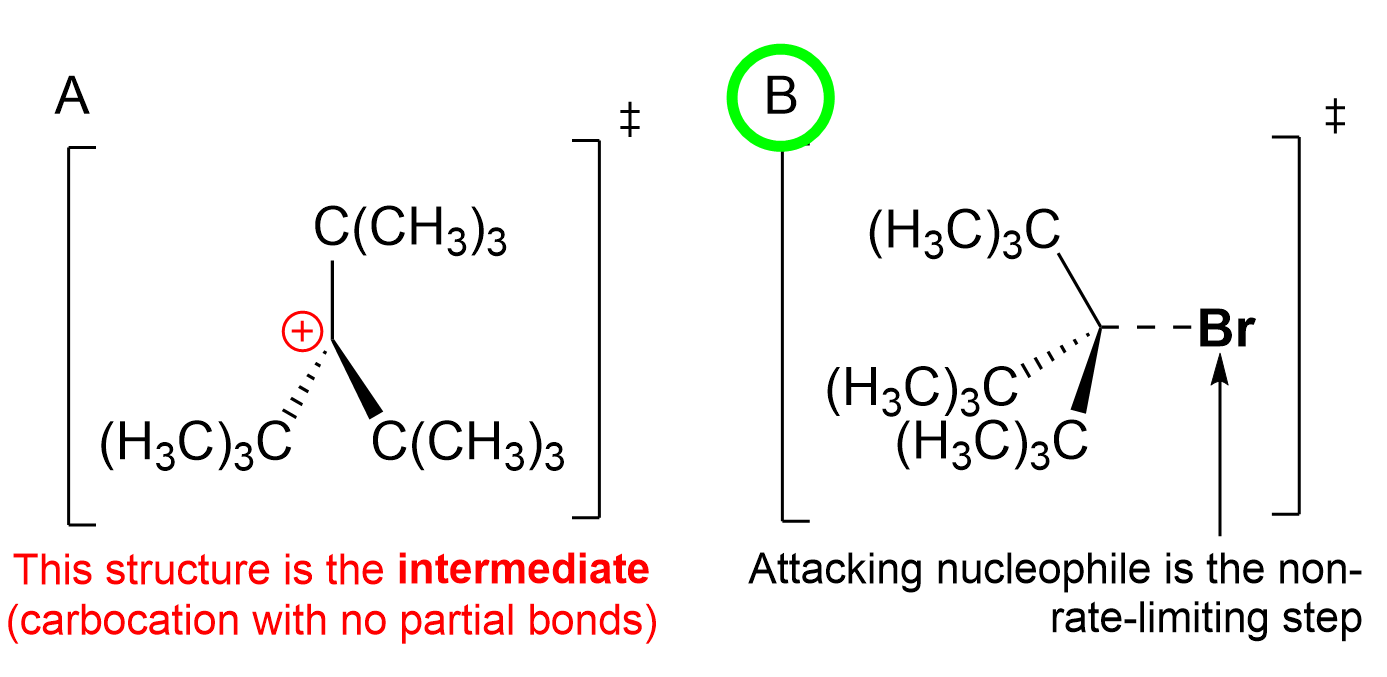
Option C is incorrect, as it shows the transition state of the first step, with the Cl leaving the molecule. This is seen by the dashed line to Cl. This first step is considered the slowest step of the reaction, and thus, it is rate-limiting.
Option D is incorrect, as it shows two partially formed bonds to both Cl and Br. Recall that SN1 reactions occur via a two-step mechanism where the leaving group departs before the carbocation is attacked by a nucleophile. Due to this mechanism, it is not possible for the transition state to contain two partially formed bonds, as one happens before the other. Thus, the chlorine should be fully dissociated from the molecule and not have a partial bond to the central carbon.
Chapter 3.1.4 – SN1 VS SN2
1. Indicate the false statement concerning organic reactions:
A. In the reaction of H2O with ClC(CH3)3, doubling [ClC(CH3)3] will not double the rate of reaction.
B. SN1 reactions of tertiary alkyl halides are faster than SN1 reactions of secondary alkyl halides.
C. The reaction of HO− with ClCH2CH3 does not involve a carbocation intermediate.
D. SN2 reactions become nine times faster when [nucleophile] and [electrophile] both increase by a factor of three
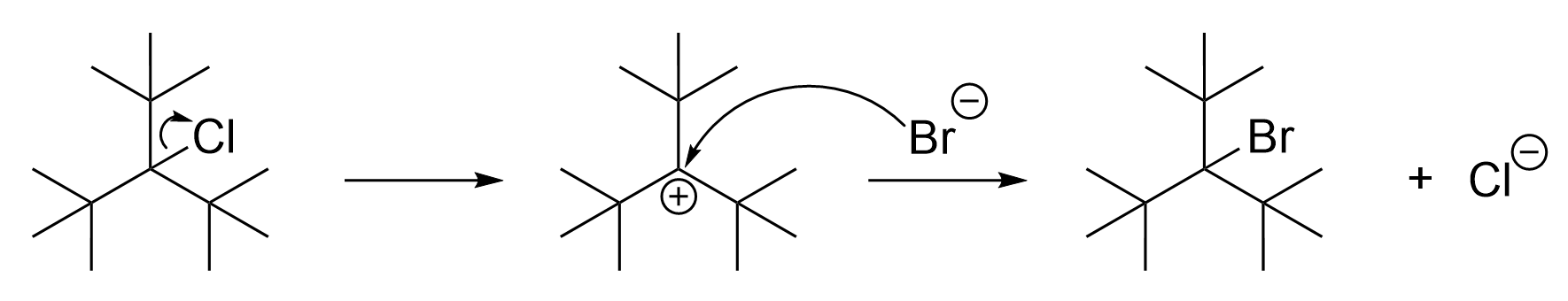
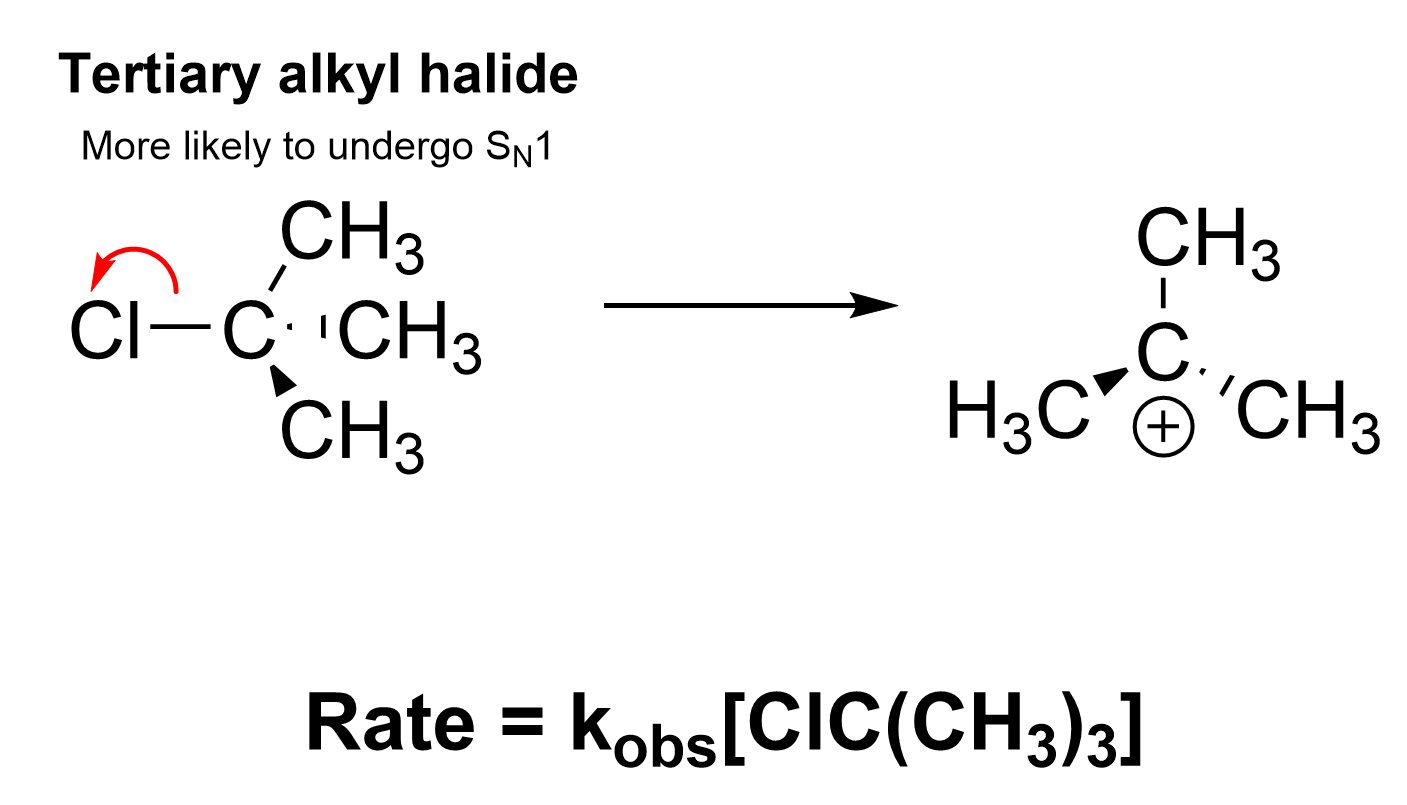
The correct option in this question is Option A. Before thinking about the rate law, you must first consider whether ClC(CH3)3 would undergo an SN1 or SN2 reaction. ClC(CH3)3 is a tertiary alkyl halide, as the carbon bonded to Cl has three alkyl groups around it. It is easier to see if drawn out, as seen below. As an SN1 reaction mechanism, the rate law is only influenced by the alkyl halide, as it is the rate-limiting step. Because of this, doubling the concentration of ClC(CH3)3, the alkyl halide, would double the rate of reaction. Therefore, this statement is false and is the correct answer.
Options B, C and D are incorrect as they are all true statements. SN1 reactions always occur faster with tertiary alkyl halides compared to secondary alkyl halides due to the stabilization of the carbocation from the surrounding alkyl groups. In option C, the molecule ClCH2CH3 is a primary alkyl halide, and thus, will go through the SN2 process. Recall that SN2 reactions do not go through a carbocation intermediate as the steps happen simultaneously, with the nucleophile attacking to kick out the leaving group. For option D, we know that the rate law of an SN2 reaction is rate = kobs[Alkyl halide][Nucleophile]. Thus, increasing both by a factor of three would make the reaction nine times faster.

