Experiment 9: Organic Chemistry Part I
Table of Contents
Introduction
| Table of Contents | next section >> |
This laboratory will serve as an introduction to some organic reactions, as well as laboratory techniques used in organic chemistry. There is also very useful information the special techniques section for both experiments. Acetylsalicylic acid (Aspirin) is traditionally synthesized by the acid-catalysed reaction of Salicylic acid with acetic anhydride (Figure 1).
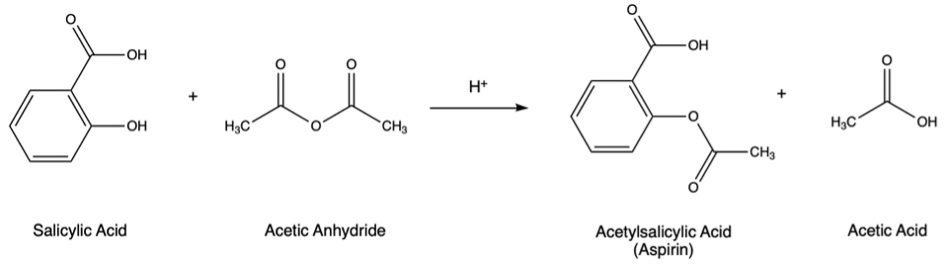
Figure 1 – Acid-catalyzed reaction of salicylic acid with acetic anhydride to form acetylsalicylic acid (Aspirin)
This reaction is an example of acetylation, the substitution of an acetyl group (O=CCH3) for a hydrogen atom. A key concept in this reaction and many others in organic chemistry is the flow of electrons between molecules. Molecules which donate electrons are called nucleophiles (nucleus loving) and are Lewis bases. Molecules which accept electrons are called electrophiles (electron loving) and are Lewis acids. Nucleophilic functional groups will donate a pair of electrons to electrophilic functional groups to form a new covalent bond. Being able to identify the nucleophilic and electrophilic functional groups of a molecule is an essential skill for organic chemists and in the report for this experiment you will be asked to identify the nucleophile and electrophile for the reaction between salicylic acid and acetic anhydride.
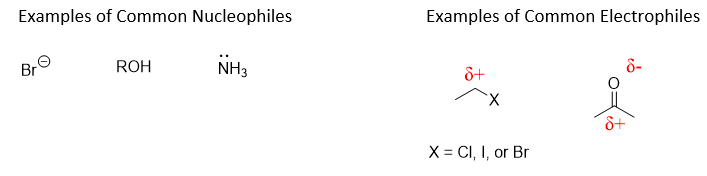
| This lab will also serve as a demonstration of recrystallization as a purification technique (see here). An important procedural note: follow the steps for the recrystallization procedure very carefully in this experiment. Addition of too much recrystallization solvent can result in low or zero yield of recrystallized product. |
Materials
| Table of Contents | << previous section | next section >> |
| Reagent | Hazard Statements | |
| Acetic Anhydride |    |
Flammable liquid and vapour.
Harmful if swallowed or if inhaled. Causes severe skin burns and eye damage. |
| Salicylic Acid |    |
Harmful if swallowed.
Causes serious eye damage. Suspected of damaging fertility or the unborn child. |
| Ethanol |   |
Highly flammable liquid and vapour.
Causes serious eye irritation. |
| Acetone |   |
Highly flammable liquid and vapour.
Causes serious eye irritation. |
| Glassware | Quantity |
| Beaker, 100 mL | 1 |
| Beaker, 250 mL | 1 |
| Beaker, 400 mL | 1 |
| Flask, Erlenmeyer, 125 mL | 1 |
| Filter flask | 1 |
| Funnel, Buchner | 1 |
| Vacuum tubing | 1 |
| Glass stir rod | 1 |
Pre-Lab Work
| Table of Contents | << previous section | next section >> |
A minimum of 24 hours before the start of your lab period:
- Watch the pre-lab video
- Complete and submit the pre-lab quiz for Experiment 9 on Avenue (under Assessments > Quizzes)
- Prepare a flowchart (see here) of the experimental steps in your laboratory notebook. Take photos of these pages and upload them to the Exp 9 Pre-Lab Assignment folder (Assessments > Assignments)
- Prepare an empty table of observations for materials/reagents you will be using and making in this lab and submit it along with your flowchart on Avenue.
Procedure
| Table of Contents | << previous section | next section >> |
Students are to work individually. No chemicals are to be placed down the sink. Use the appropriate waste containers.
| Caution: Acetic anhydride is a toxic and irritating substance. Avoid inhalation or contact with the skin. Wear gloves. Do not used graduated cylinders, as there are plastic pipettes with volume measurements attached to reagent bottles. All work is to be done in the fume hood.
General comment on cleaning organic chemicals: No waste is to be put down the drain. All glassware must be rinsed with a small amount of acetone into the organic waste before washing in the sink. (Water will not remove any residual organic compounds from dirty glassware, in most cases, once rinsed with acetone, glassware is considered clean and does not need further washing in the sink). |
Be sure to take observations while proceeding
- Students are to work individually.
- Before putting on gloves, fill your wash bottle with DI water and place it in an ice bath. Also prepare a hot-water bath (~1/2 filled 400 mL beaker) before measuring any chemicals.
- Measure accurately ~ 2 g (± 0.1 g) of salicylic acid and place it in a DRY 125 mL Erlenmeyer flask. When your hot water bath is ready (i.e., it is boiling), add as much acetic anhydride to your flask so that it just covers the salicylic acid. Swirl the flask to wet the salicylic acid. If the mixture does not swirl easily, then add a bit more acetic anhydride.
- Add 5 drops of conc. H2SO4 to the mixture and heat the flask in the hot water bath for 5-10 minutes which has been brought to a boil. Be careful not to overheat the flask as it will decompose the acetic anhydride.
- Carefully remove the flask from the hot water bath, and to the reaction add 10 mL of pre-chilled distilled water. Cool the reaction in an ice bath to allow the aspirin crystals to form. Once you no longer see new crystals form, then you can move onto the next step.
- If crystals still do not appear after the flask has already cooled down (4-10ºC), induce crystallization by scratching the inside of the reaction flask with a clean stirring rod and continue to cool the flask. Note: Do not apply this method of inducing crystallization during the second recrystallization process below.
- If an oily layer appears, reheat the flask until the oil disappears, then cool the flask.
- If the solution appears yellowish in colour, it is likely that the acetic anhydride has decomposed. In this case, repeat the addition of 4-5 mL of acetic anhydride followed by 5 drops of conc. H2SO4 only. (Do NOT add more water.) Swirl and reheat the flask for 5-10 minutes before cooling in an ice-water bath.
- Set up the vacuum filtration apparatus described here (make sure the filter flask is clean in case the contents need to be salvaged). Filter the aspirin. Quantitatively transfer the aspirin crystals remaining in the flask into the funnel by rinsing the flask with small portions of cold (i.e., pre-chilled) distilled water. Then proceed to wash the aspirin in the filter funnel with 15 mL of cold water. Allow the aspirin to dry in the filter funnel for a few minutes. To enable efficient drying, use a clean flat-tipped spatula to loosen and break off any crystals present in clumps, while being careful not to scratch and break the filter paper. Isolate any significant amounts of aspirin that has transferred or crystallized in the filter flask.
- Transfer the dried crystals into a clean 100- or 250-mL beaker.
- Collect 10 mL of ethanol in a separate 100 mL beaker. Warm it on the hot plate just prior to using as it is very volatile. Once warmed, add ethanol dropwise using a Pasteur pipette until the crystals are just dissolved (i.e., add slowly and swirl often). In most cases, you will not need to use the full 10 mL. If some crystals remain undissolved, then gently warm the beaker containing the ethanol-crystal mixture on the hot plate (~60ºC).
- Once all crystals have dissolved, add 30 mL of warm (~60°C) distilled water. (Tip: The water may be poured slowly and in small portions, again just to ensure that all crystals remain completely dissolved in this step.) Ensuring that the solution is clear and free of undissolved crystals, begin the recrystallization process by removing the beaker from heat and allowing the solution to cool slowly to room temperature on the benchtop, before placing the beaker into an ice-water bath. (Be careful not to disturb the beaker as much as possible while cooling—i.e., do not shake, swirl, or mix the solution). After 10 minutes or longer, crystals will slowly appear.
- Collect the aspirin crystals by suction filtration and wash with 2 x 10 mL of cold water. Allow the crystals to dry in the filter funnel for a few minutes, then remove and spread the aspirin on filter paper and allow to air dry until leaving the laboratory.
- Label a sample test tube with your initials and ID number. Record the mass of this labelled test tube in your notebook.
- Transfer the aspirin crystals to the sample tube and give this to your teaching assistant for storage until the next experiment.
- Dispose of chemical waste in the appropriate waste containers (see here).
- Rinse glassware with acetone (into a waste container) and/or distilled water and return it to your drawer.
- Wipe down your lab bench with paper towels or a j-cloth.
Report
| Table of Contents | << previous section |
The report is done individually and prepared directly in your laboratory notebook. Be sure to include all results and observations, including balanced chemical equations where appropriate.
Write out the reaction scheme for the reaction you performed in this experiment and label all reactants and products. Identify all functional groups present in the reactants and products. Identify nucleophilic and electrophilic atoms/functional groups of this reaction, and briefly explain your reasoning.
Have your TA sign and date every page of your data for this experiment. Within 1 hour of the end of your lab period, upload a single pdf of the images of your data pages and report to the appropriate Avenue Assignment folder (Assessments > Assignments > Experiment 9 Report). We recommend using the Microsoft Lens app for quick preparation of the final pdf.
