Experiment 3: Determination of an Equilibrium Constant for a Chemical Reaction
Table of Contents
Introduction
| Table of Contents | next section >> |
Chemical reactions are often reversible and when the rate of the forward and reverse reactions is the same, chemical equilibrium is observed. Reaction mixtures at equilibrium obey the Law of Chemical Equilibrium, which imposes certain conditions on the concentration of reactants and products and is ultimately defined by the equilibrium constant for the reaction.
In this experiment the reaction between Fe3+ ions and thiocyanate ions, SCN–, will be studied. When the solutions containing both ions are mixed together, a deep red colour, attributed to the FeSCN2+ ion, appears according to the equation:
![]()
The reaction goes forward to some extent and at equilibrium the concentration of all species is expressed by:
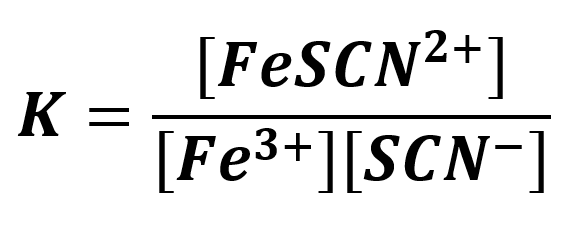
The purpose of this experiment is to find the value of K for this reaction which will be done for several concentrations of reactants. In the reaction we will mix a known concentration of KSCN and Fe(NO3)3. From the initial concentrations of the reactants and the concentration of the formed FeSCN2+ product, we will calculate the equilibrium concentrations of all the species and subsequently the equilibrium constant. The concentration of FeSCN2+ will be determined using a spectrophotometer, the Genesys 20.
Materials
| Table of Contents | << previous section | next section >> |
| Reagent | Hazard Statements | |
| Potassium thiocyanate (0.00200 M) |   |
Harmful if swallowed
Harmful in contact with skin Harmful if inhaled |
| Iron (III) Nitrate (0.0000 – 0.200 M) |   |
May cause eye irritation
May cause skin irritation May cause irritation of the digestive tract May cause respiratory tract irritation |
| Glassware | Quantity |
| Beaker, 250 mL | 2 |
| Beaker, 100 mL | 2 |
| Flask, volumetric, 50 mL | 3 |
| Funnel, poly | 3 |
| Tube, culture, 13 x 100 | 6 |
| Tube, culture, 16 x 150 | 5 |
| Meniscus reader | 1 |
Pre-Lab Work
| Table of Contents | << previous section | next section >> |
A minimum of 24 hours before the start of your lab period:
- Watch the pre-lab video
- Complete and submit the pre-lab quiz for Experiment 3 on Avenue (under Assessments > Quizzes).
- Prepare a flowchart (see here) of the experimental steps in your laboratory notebook. Uploaded images of your flowchart to the Exp 3 Pre-Lab Assignment folder (Assessments > Assignments)
- Prepare any data tables that you think will help to keep your results organized. You do not need to submit these to Avenue as part of your pre-lab assignment, but they do help you to get the lab completed on time and with higher marks overall.
Procedure
| Table of Contents | << previous section | next section >> |
Before starting the experiment, read the section about glassware used in volumetric analysis and the section on spectrophotometers found in the techniques chapter. Students will work in pairs and two pairs will share a spectrophotometer. Each pair must perform all portions of the experiment. Do not forget to put your lab partner’s name in your report.
Part A. Calibration Curve for Spectrophotometer
Using a spectrophotometer to determine an unknown concentration of FeSCN2+ first requires one to determine the relationship between absorbance and concentration A = m y [Conc] (determine my). In this part, by using a huge excess of iron (III) nitrate, the reaction is forced to such an extent that virtually all SCN– ions have reacted to form FeSCN2+. Knowing the concentrations of the KSCN used and the absorbance values, one can convert the readings taken during the experiment to correspond to the concentration of FeSCN2+. Use a wavelength of 447 nm.
- Collect ~50 mL of 0.00200 M KSCN (aq) using a 100 mL beaker. After rinsing and conditioning the burette, half-fill the burette to the ~25 mL mark with KSCN solution. This is labelled burette a. Both you and your partner will use this solution in Parts A and B.
- Collect ~175 mL of 0.200 M Fe(NO3)3 (aq) using a 250 mL beaker. Prepare three 50.00 mL volumetric flasks. Rinse them with distilled water and then several times with a very small amount of 0.200 M ferric nitrate solution. Label them appropriately.
- From burette a, deliver 1.0 mL, 2.0 mL, and 3.0 mL of KSCN solution to the 1st, 2nd, and 3rd flasks, respectively
- Fill all three flasks to the mark with 0.200 M Fe(NO3)3. Invert (do not shake!) each flask several times, so that the solutions inside will mix well.
- Standardize the spectrophotometer using DI water as the calibration blank solution (see page xi for more details on use of the spectrophotometer).
- Rinse the cuvette 3x with small amounts of the solution you are measuring before filing it ¾ full of the solution. Clean and wipe the outside of the cuvette with a KimWipe. Measure the absorption of the solution by placing the cuvette in the spectrophotometer and gently closing the lid of the sample compartment. Repeat this step for the other two solutions. Rinse the test cuvettes with DI water before proceeding to the next section.
Part B. Equilibrium Constant for a Chemical Reaction
All glassware should be clean and all measurements done as precisely as possible.
1. Prepare two more burets: burettes b and c. Your partner should have already prepared burette a with 0.00200 M KSCN in Part A. Half-fill burette b with 0.00200 M Fe(NO3)3 solution. Fill burette c with distilled water.
2. Label 3 clean and dry test tubes from 1 to 3.
3. From burette a, add 0.00200 M KSCN to each test tube as shown in the table below.
4. From burette b, add 0.00200 M Fe(NO3)3 to each test tube as shown in the table below.
5. From burette c, add DI water to each test tube as shown in the table below.
| Regent | Test Tube No. | ||
| 1 | 2 | 3 | |
| Vol. 0.00200 M KSCN (mL | 2.00 | 3..00 | 4.00 |
| Vol. 0.00200 M Fe(NO3)3 (mL) | 5.00 | 5.00 | 5.00 |
| Vol. H2O (mL) | 3.00 | 2.00 | 1.00 |
6. Mix the solution in each test tube well using a stirring rod. Remember to clean it before mixing the next solution.
7. Transfer a portion of each solution into a small, clean spectrophotometer test tube that has been rinsed 3x with a small amount of your solution. The test tube should be 3/4 full, clean, and wiped on the outside with a Kimwipe.
8. Measure the absorbance of each of the solutions in the test tubes. Make sure the lid of the sample compartment is closed when measurements are recorded.
9. When all solutions have been measured, discard the solutions in the appropriate waste containers. Rinse the glassware thoroughly with DI water.
10. Wipe down your lab bench with paper towels or a j-cloth and ensure all glassware is put away in your draw-er.
Report
| Table of Contents | << previous section |
Include all the results and answer any questions appearing in the procedure as well as the items listed below. Consult the report-writing guidelines here, for additional details on report requirements.
You may discuss your reports with your partner, but you must prepare and submit INDIVIDUAL reports. Plagiarism offences are taken very seriously and will be brought to the Academic Integrity Office.
Calculations For Part A
In Part A, by using a huge excess of ferric nitrate, we forced the reaction to go to the right to such an extent that almost all the SCN– ions reacted to form FeSCN2+. As a result, each mole of SCN– ions added to the solution was converted to a mole of FeSCN2+ions.
Calculate the initial [SCN–] in each reaction mixture. Consider that 1.0 mL of KSCN (or 2.0 mL or 3.0 mL) from burette a was diluted to a final volume of 50.00 mL. The concentration of the coloured FeSCN2+ ions that were formed is equal to the initial concentration of SCN– ions.
Graph
Plot the absorbance, A (y-axis) versus concentration of FeSCN2+ (x-axis) and draw the line-of-best-fit passing through the origin. Determine the slope of this line using the line of best fit (NOT the actual data points). Consult the graphing directions for correct format.
Calculations For Part B: Calculating The Concentrations of Ions Present In Equilibrium
- From your calibration curve determine the concentration of FeSCN2+ ions formed at equilibrium in each of the three test tubes from Part B.
- Calculate the equilibrium concentration of SCN– and Fe3+ ions.
- Calculate the value of K for each trial but only show one sample calculation using data from trial 1.
- Determine the aver-age value of K from all three trials.
Discussion Of The Results
In addition to a concise statement of results, consider the following discussion points:
- Are your calculated values of the equilibrium constant the same for all the samples? If not, why?
- What are the factors that can change the value of the equilibrium constant?
Report Submission
Have your TA sign and date every page of your data for this experiment. Within 1 hour of the end of your lab period, upload a single pdf of the images of your data pages and report to the appropriate Avenue Assignment folder (Assessments > Assignments > Experiment 3 Report). We recommend using the Microsoft Lens app for quick preparation of the final pdf.
