Experiment 5: Electrochemistry Inquiry
Table of Contents
Introduction
| Table of Contents | next section >> |
Students will need a textbook or a list of reduction potentials during the lab. A calculator is also necessary for this experiment.
This experiment is inquiry based and will focus on procedure and problem-solving development by creating simple galvanic electrochemical cells.
During an oxidation–reduction reaction, electrons are transferred between two chemical species. As an example, if aluminum metal is added to a solution of copper(II) sulphate, electron transfer will occur from aluminum (reducing agent) to Cu2+ (oxidizing agent) through the following reaction:
2 Al(s) + 3 CuSO4(aq) → 3 Cu(s) + Al2(SO4)3(aq)
If the reaction takes place entirely in one vessel (the aluminum is added directly to the copper sulphate solution), while electrons are being transferred, it becomes difficult to harness the movement of electrons. However, the reaction above can be considered in two half-cells, showing the transfer of six electrons.
(Al → Al3+ + 3e–) x 2 oxidation half-reaction
(Cu2+ + 2e– → Cu) x 3 reduction half-reaction
If the two half-cell reactions are physically separated in two vessels, the flow of electrons can be harnessed to produce an electric current and potential (as seen below, using an iron/copper cell; Figure 14). The resultant is a galvanic electrochemical cell.
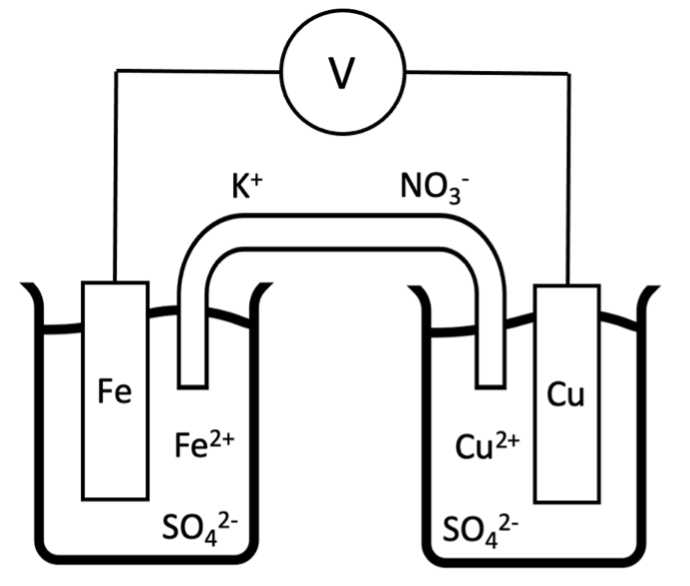
A simple half-cell consists of a piece of metal, called an electrode, immersed in a solution containing cations of the metal. A potential difference is observed when the electrodes of two different half-cells are connected by a wire, and the compartments joined by a porous membrane or salt bridge. The salt bridge allows ions to be transferred between the two half-cells to maintain electrical neutrality, but prevents the two solutions from mixing. To represent an electrochemical cell in a simplistic manner, a cell diagram (shown below) can be used:
Fe(s) | Fe2+(aq,1.00 M) || Cu2+(aq,1.00 M) | Cu(s)
The electrode on the left, where oxidation occurs, is called the anode. On the right, the process of reduction takes place at the cathode. The double vertical lines denote the salt bridge. Single vertical lines indicate a change of state. States and concentrations must be included to ensure the electrochemical cell is described completely.
The potential difference between the two electrodes, measured in volts, is called the cell potential Ecell; when the concentrations are under standard conditions (1 mol L–1, 1 atm and temperature is the temperature of interest, and is not implied to be 298 K for “standard conditions”—same for delta H, although data are often quoted at 298 K) the potential is termed the standard cell potential, E°cell.
To calculate a cell potential, standard reduction potentials are needed, which are tabulated in the textbook. The standard hydrogen half-cell, also referred to as the standard hydrogen electrode (SHE), is the standard to which all other half-cell potentials are compared. The standard hydrogen half-cell potential is arbitrarily assigned a value of 0.00 V. All standard half-reactions are conveniently listed as reduction half-reactions, and their standard potentials are given as reduction potentials, E°red.
Species having large positive standard reduction potentials are very strong oxidizing agents (example fluorine: F2(g) + 2e– → 2F–(aq); E°red = + 2.866 V). Those having large, negative values are strong reducing agents when considering the reverse reaction (example lithium metal: Li+(aq) + e– → Li(s); E°red = −3.040 V).
To calculate a standard cell potential both standard reduction potentials of the half-cell reactions must be considered. As an example, use the electrochemical cell described by the following cell diagram:
Fe(s) | Fe2+(aq,1.00 M) || Cu2+(aq,1.00 M) | Cu(s)
The two half-reactions with standard reduction potentials are:
Fe2+ + 2e– → Fe E°red = –0.440 V
Cu2+ + 2e– → Cu E°red = 0.340 V
According to the cell diagram, the first half-reaction takes place in the opposite direction (Fe is oxidized to Fe2+) and is therefore the anode. Then the standard cell potential becomes:
E°cell = E°right (cathode) − E°left (anode) = (0.340 V – (−0.440 V) = + 0.78 V.
To find out if an oxidation–reduction reaction proceeds spontaneously under standard conditions, in the direction written, calculate E°cell = E°right (cathode) − E°left (anode) for the reaction as written. A positive value of the standard cell potential shows that the reaction occurs spontaneously in the direction written. If the calculated E°cell is negative, the reverse reaction, which has a positive E°cell, is spontaneous.
When standard conditions (1 mol L–1, 1 atm) are not met or the system is not at equilibrium, the cell potential Ecell varies from the standard cell potential E°cell and can be calculated using the Nernst equation (below). Any Ecell can be obtained, providing E°cell is known, simply by changing concentrations or pressures of the species in each half-cell.
Ecell = E°cell – (RT/nF) ln Q
Ecell is the cell potential under non-standard conditions,
E°cell is the standard cell potential (calculated from the standard reduction potentials),
R is the gas constant (8.314 J K–1 mol–1),
T is the temperature in degrees Kelvin,
F is the Faraday constant (96485 C mol–1),
n is the number of electrons taking part in the reaction, and
Q represents the reaction quotient,
Q = [concentration or pressure of products] / [concentration or pressure of reactants]
Materials
| Table of Contents | << previous section | next section >> |
| Reagent | Hazard Statements | |
| Copper (II) Sulfate (0.05 M) |    |
Irritant, skin and eye
Acute hazards to the aquatic environment May cause damage to hematopoietic system, kidneys, liver and/or stomach through prolonged exposure or if swallowed |
| Zinc Sulfate (0.05 M) |   |
Causes serious eye irritation
Very toxic to aquatic life |
| Tin (II) Chloride (0.05 M) |    |
Harmful if swallowed or inhaled
Causes severe skin burns and eye damage May cause an allergic skin reaction May cause respiratory irritation May cause damage to organs through prolonged or repeated exposure |
| Glassware | Quantity |
| Voltmeter | 1 |
| 2.00 mL graduated pipet | 2 |
| 50.00 mL volumetric flask | 2 |
| 50 mL beaker | 6 |
| Metal plates (Cu, Zn, Sn) | 1 each |
Pre-Lab Work
| Table of Contents | << previous section | next section >> |
A minimum of 24 hours before the start of your lab period:
- Watch the pre-lab video
- Complete and submit the pre-lab quiz for Experiment 5 on Avenue (under Assessments > Quizzes).
- Prepare a flowchart (see here) of the experimental steps in your laboratory notebook. Uploaded images of your flowchart to the Exp 5 Pre-Lab Assignment folder (Assessments > Assignments)
- Prepare any data tables that you think will help to keep your results organized. You do not need to submit these to Avenue as part of your pre-lab assignment, but they do help you to get the lab completed on time and with higher marks overall.
Procedure
| Table of Contents | << previous section | next section >> |
General Instruction For The Construction Of An Electrochemical Cell
Students will work in pairs. Clean a strip of metal A and a strip of metal B (use emery paper and work in the fumehood). To a clean 50 mL beaker pour 20 mL of a solution of metal A2+(aq). Repeat with a second 50 mL beaker and a solution of metal B2+(aq). The levels of solutions in the two beakers should be equal. Connect the two beakers by a salt bridge, which is a 1.0 M KNO3 solution in water and agar, which is already prepared. Complete the circuit by connecting the voltmeter. The clips connecting the metal to the voltmeter must not contact the solution. Once the voltmeter is on the right setting, a reading will appear.
Do not forget to gently rinse (with distilled water) the outside of the salt bridge each time it is placed in a new solution. When not in use, the salt bridge must remain in the solution of potassium nitrate.
Part A: Creation of a cell using zinc and copper
Using the description above, construct an electrochemical cell based on Zn(s)/Zn2+(0.05 M) and Cu2+(0.05 M)/Cu(s). Measure the cell potential and compare to the theoretical cell potential by calculating a percent error. Also include a cell diagram (as seen in the introduction).
Part B: Creation of an electrochemical cell based on a concentration gradient
Make an electrochemical cell based on two Cu2+(aq)/Cu(s) half-cells. One should have a concentration of 0.05 M, the other 0.00005 M. You will need to dilute the 0.05 M solution using both volumetric flasks and serial dilutions to obtain the 0.00005 M solution. Clearly show all calculations required for the dilution. Measure the potential and compare to the theoretical value by calculating the percent error. For the theoretical potential you will need to use the Nernst equation for the calculation. Also include a cell diagram (as seen in the introduction).
Part C: Design and creation of an electrochemical cell from a given cell potential
The teaching assistant will assign you a target cell potential. You will be required to construct an electrochemical cell that will produce a cell potential as close as possible to the assigned potential. You may use any combination of metals and solutions given (Zn, Cu, Sn) however, one combination will work best – you may need to dilute one of the provided metal solutions. You must show your TA the reading on the voltmeter when the cell has been constructed. It may not be possible to produce the exact cell potentials given but make the best effort possible in the time frame given. Compare the measured cell potential to the theoretical cell potential by calculating a percent error. Also include a cell diagram (as seen in the introduction). Dispose of chemical waste into the appropriate waste containers (see page iv). Rinse glassware with distilled water and return it to your drawer.
- Wipe down your lab bench with paper towels or a j-cloth.
- Have your TA sign and date every page of your data for this experiment.
Report
| Table of Contents | << previous section |
You may discuss your reports with your partner, but you must prepare and submit INDIVIDUAL reports. Plagiarism offenses are taken seriously and will be brought to the Academic Integrity Office.
Suggest reasons as to why obtaining the assigned potential in Part C was difficult (if that was the case).
Report Submission
Have your TA sign and date every page of your data for this experiment. Within 1 hour of the end of your lab period, upload a single pdf of the images of your data pages and report to the appropriate Avenue Assignment folder (Assessments > Assignments > Experiment 5 Report). We recommend using the Microsoft Lens app for quick preparation of the final pdf.
