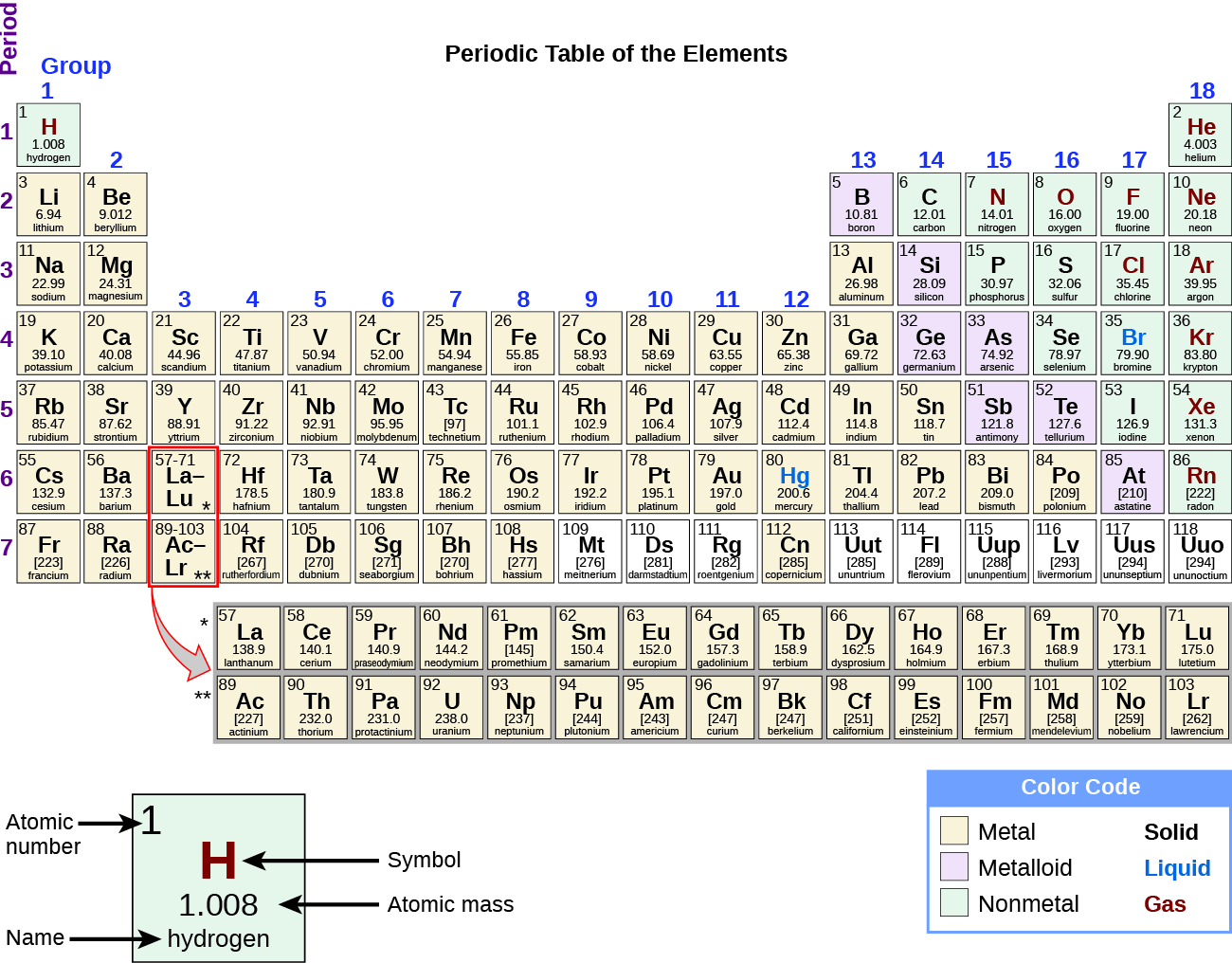
Appendix A: The Periodic Table
Graphical version of the periodic table of the elements. The 18 columns are labeled “Group” and the 7 rows are labeled “Period.” Below the table to the right is a box labeled “Color Code” with different colors for metals, metalloids, and nonmetals, as well as solids, liquids, and gases. To the left of this box is an enlarged picture of the upper-left most box on the table. The number 1 is in its upper-left hand corner and is labeled “Atomic number.” The letter “H” is in the middle in red indicating that it is a gas. It is labeled “Symbol.” Below that is the number 1.008 which is labeled “Atomic Mass.” Below that is the word hydrogen which is labeled “name.” The color of the box indicates that it is a nonmetal. Each element will be described in this order: atomic number; name; symbol; whether it is a metal, metalloid, or nonmetal; whether it is a solid, liquid, or gas; and atomic mass.Below, please find the periodic table of the elements in table/text format.
Period (Row)
Group (Column)
Atomic Number
Symbol
Name
Atomic Mass
State of Matter at Room Temperature
Type of Element
Family
Common Ion
Valence Electrons
Outer Shell Electron Configuration
Electronegativity Values
1
1
1
H
Hydrogen
1.008
gas
nonmetal
–
H+
1
1s1
2.1
1
18
2
He
Helium
4.003
gas
nonmetal
Noble gas
no ion
2
1s2
–
2
1
3
Li
Lithium
6.94
solid
metal
Alkali metal
Li+
1
2s1
1.0
2
2
4
Be
Beryllium
9.01
solid
metal
Alkaline earth metal
Be2+
2
2s2
1.5
2
13
5
B
Boron
10.81
solid
metalloid
–
3
2s2 2p1
2.0
2
14
6
C
Carbon
12.01
solid
nonmetal
–
C4-
4
2s2 2p2
2.5
2
15
7
N
Nitrogen
14.01
gas
nonmetal
Pnictogen
N3-
5
2s2 2p3
3.0
2
16
8
O
Oxygen
16.00
gas
nonmetal
Chalcogen
O2-
6
2s2 2p4
3.5
2
17
9
F
Fluorine
19.00
gas
nonmetal
Halogen
F–
7
2s2 2p5
4.0
2
18
10
Ne
Neon
20.18
gas
nonmetal
Noble gas
no ion
8
2s2 2p6
–
3
1
11
Na
Sodium
22.99
solid
metal
Alkali metal
Na+
1
3s1
0.9
3
2
12
Mg
Magnesium
24.30
solid
metal
Alkaline earth metal
Mg2+
2
3s2
1.2
3
13
13
Al
Aluminum
26.98
solid
metal
–
Al3+
3
3s2 3p1
1.5
3
14
14
Si
Silicon
28.08
solid
metalloid
–
4
3s2 3p2
1.8
3
15
15
P
Phosphorus
30.97
solid
nonmetal
Pnictogen
P3-
5
3s2 3p3
2.1
3
16
16
S
Sulfur
32.06
solid
nonmetal
Chalcogen
S2-
6
3s2 3p4
2.5
3
17
17
Cl
Chlorine
35.45
gas
nonmetal
Halogen
Cl–
7
3s2 3p5
3.0
3
18
18
Ar
Argon
39.79
gas
nonmetal
Noble gas
no ion
8
3s2 3p6
–
4
1
19
K
Potassium
39.10
solid
metal
Alkali metal
K+
1
4s1
0.8
4
2
20
Ca
Calcium
40.08
solid
metal
Alkaline earth metal
Ca2+
2
4s2
1.0
4
3
21
Sc
Scandium
44.96
solid
metal
Transition metal
4s2 3d1
1.3
4
4
22
Ti
Titanium
47.87
solid
metal
Transition metal
4s2 3d2
1.5
4
5
23
V
Vanadium
50.94
solid
metal
Transition metal
4s2 3d3
1.6
4
6
24
Cr
Chromium
52.00
solid
metal
Transition metal
Cr3+ Cr6+
4s1 3d5
1.6
4
7
25
Mn
Manganese
54.94
solid
metal
Transition metal
Mn2+
4s2 3d5
1.5
4
8
26
Fe
Iron
55.85
solid
metal
Transition metal
Fe2+ Fe3+
4s2 3d6
1.8
4
9
27
Co
Cobalt
58.93
solid
metal
Transition metal
Co2+
4s2 3d7
1.9
4
10
28
Ni
Nickel
58.69
solid
metal
Transition metal
Ni2+
4s2 3d8
1.9
4
11
29
Cu
Copper
63.55
solid
metal
Transition metal
Cu+ Cu2+
4s1 3d10
1.9
4
12
30
Zn
Zinc
65.38
solid
metal
Transition metal
Zn2+
4s2 3d10
1.6
4
13
31
Ga
Gallium
69.72
solid
metal
–
4s2 3d10 4p1
1.6
4
14
32
Ge
Germanium
72.63
solid
metalloid
–
4s2 3d10 4p2
1.8
4
15
33
As
Arsenic
74.92
solid
metalloid
Pnictogen
As3-
4s2 3d10 4p3
2.0
4
16
34
Se
Selenium
78.97
solid
nonmetal
Chalcogen
Se2-
4s2 3d10 4p4
2.4
4
17
35
Br
Bromine
79.90
liquid
nonmetal
Halogen
Br–
4s2 3d10 4p5
2.8
4
18
36
Kr
Krypton
83.80
gas
nonmetal
Noble gas
no ion
4s2 3d10 4p6
–
5
1
37
Rb
Rubidium
85.47
solid
metal
Alkali metal
Rb+
5s1
0.8
5
2
38
Sr
Strontium
87.62
solid
metal
Alkaline earth metal
Sr2+
5s2
1.0
5
3
39
Y
Yttrium
88.91
solid
metal
Transition metal
5s2 4d1
1.2
5
4
40
Zr
Zirconium
91.22
solid
metal
Transition metal
5s2 4d2
1.4
5
5
41
Nb
Niobium
92.91
solid
metal
Transition metal
5s1 4d4
1.6
5
6
42
Mo
Molybdenum
95.95
solid
metal
Transition metal
5s1 4d5
1.8
5
7
43
Tc
Technetium
98.91
solid
metal
Transition metal
5s1 4d6
1.9
5
8
44
Ru
Ruthenium
101.1
solid
metal
Transition metal
5s1 4d7
2.2
5
9
45
Rh
Rhodium
102.9
solid
metal
Transition metal
5s1 4d8
2.2
5
10
46
Pd
Palladium
106.4
solid
metal
Transition metal
Ag+
4d10
2.2
5
11
47
Ag
Silver
107.9
solid
metal
Transition metal
Cd2+
5s1 4d10
1.9
5
12
48
Cd
Cadmium
112.4
solid
metal
Transition metal
5s2 4d10
1.7
5
13
49
In
Indium
114.8
solid
metal
–
5s2 4d10 5p1
1.7
5
14
50
Sn
Tin
118.7
solid
metal
–
5s2 4d10 5p2
1.8
5
15
51
Sb
Antimony
121.8
solid
metalloid
Pnictogen
5s2 4d10 5p3
1.9
5
16
52
Te
Tellurium
127.6
solid
metalloid
Chalcogen
Te2-
5s2 4d10 5p4
2.1
5
17
53
I
Iodine
126.9
solid
nonmetal
Halogen
I–
5s2 4d10 5p5
2.5
5
18
54
Xe
Xenon
131.3
gas
nonmetal
Noble gas
no ion
5s2 4d10 5p6
–
6
1
55
Cs
Cesium
132.9
solid
metal
Alkali metal
Cs+
6s1
0.7
6
2
56
Ba
Barium
137.3
solid
metal
Alkaline earth metal
Ba2+
6s2
0.9
6
3
57
La
Lanthanum
138.9
solid
metal
Lanthanide
6s2 5d1
1.0-1.2
6
n/a
58
Ce
Cerium
140.1
solid
metal
Lanthanide
6s2 4f2
1.0-1.2
6
n/a
59
Pr
Praseodymium
140.9
solid
metal
Lanthanide
6s2 4f3
1.0-1.2
6
n/a
60
Nd
Neodymium
144.2
solid
metal
Lanthanide
6s2 4f4
1.0-1.2
6
n/a
61
Pm
Promethium
145.0
solid
metal
Lanthanide
6s2 4f5
1.0-1.2
6
n/a
62
Sm
Samarium
150.4
solid
metal
Lanthanide
6s2 4f6
1.0-1.2
6
n/a
63
Eu
Europium
152.0
solid
metal
Lanthanide
6s2 4f7
1.0-1.2
6
n/a
64
Gd
Gadolinium
157.3
solid
metal
Lanthanide
6s2 4f7 5d1
1.0-1.2
6
n/a
65
Tb
Terbium
158.9
solid
metal
Lanthanide
6s2 4f9
1.0-1.2
6
n/a
66
Dy
Dysprosium
162.5
solid
metal
Lanthanide
6s2 4f10
1.0-1.2
6
n/a
67
Ho
Holmium
164.9
solid
metal
Lanthanide
6s2 4f11
1.0-1.2
6
n/a
68
Er
Erbium
167.3
solid
metal
Lanthanide
6s2 4f12
1.0-1.2
6
n/a
69
Tm
Thulium
168.9
solid
metal
Lanthanide
6s2 4f13
1.0-1.2
6
n/a
70
Yb
Ytterbium
173.0
solid
metal
Lanthanide
6s2 4f14
1.0-1.2
6
n/a
71
Lu
Lutetium
175.0
solid
metal
Lanthanide
6s2 4f14 5d1
1.0-1.2
6
4
72
Hf
Hafnium
178.5
solid
metal
Transition metal
6s2 4f14 5d2
1.3
6
5
73
Ta
Tantalum
180.9
solid
metal
Transition metal
6s2 4f14 5d3
1.5
6
6
74
W
Tungsten
183.8
solid
metal
Transition metal
6s2 4f14 5d4
1.7
6
7
75
Re
Rhenium
186.2
solid
metal
Transition metal
6s2 4f14 5d5
1.9
6
8
76
Os
Osmium
190.2
solid
metal
Transition metal
6s2 4f14 5d6
2.2
6
9
77
Ir
Iridium
192.2
solid
metal
Transition metal
6s2 4f14 5d7
2.2
6
10
78
Pt
Platinum
195.1
solid
metal
Transition metal
Pt2+
6s1 4f14 5d9
2.2
6
11
79
Au
Gold
197.0
solid
metal
Transition metal
Au+ Au3+
6s1 4f14 5d10
2.4
6
12
80
Hg
Mercury
200.6
liquid
metal
Transition metal
Hg2 2+ Hg2+
6s2 4f14 5d10
1.9
6
13
81
Tl
Thallium
204.4
solid
metal
–
6s2 4f14 5d10 6p1
1.8
6
14
82
Pb
Lead
207.2
solid
metal
–
6s2 4f14 5d10 6p2
1.9
6
15
83
Bi
Bismuth
209.0
solid
metal
Pnictogen
6s2 4f14 5d10 6p3
1.9
6
16
84
Po
Polonium
209
solid
metal
Chalcogen
6s2 4f14 5d10 6p4
2.0
6
17
85
At
Astatine
210
solid
metalloid
Halogen
At–
6s2 4f14 5d10 6p5
2.2
6
18
86
Rn
Radon
222
gas
nonmetal
Noble gas
no ion
6s2 4f14 5d10 6p6
–
7
1
87
Fr
Francium
223
solid
metal
Alkali metal
Fr+
7s1
0.7
7
2
88
Ra
Radium
226
solid
metal
Alkaline earth metal
Ra2+
7s2
0.9
7
3
89
Ac
Actinium
227
solid
metal
Actinide
7s2 6d1
1.1
7
n/a
90
Th
Thorium
232
solid
metal
Actinide
7s2 6d2
1.3
7
n/a
91
Pa
Protactinium
231
solid
metal
Actinide
7s2 5f2 6d1
1.4
7
n/a
92
U
Uranium
238
solid
metal
Actinide
7s2 5f3 6d1
1.4
7
n/a
93
Np
Neptunium
237
solid
metal
Actinide
7s2 5f4 6d1
1.4-1.3
7
n/a
94
Pu
Plutonium
244
solid
metal
Actinide
7s2 5f6
1.4-1.3
7
n/a
95
Am
Americium
243
solid
metal
Actinide
7s2 5f7
1.4-1.3
7
n/a
96
Cm
Curium
247
solid
metal
Actinide
7s2 5f7 6d1
1.4-1.3
7
n/a
97
Bk
Berkelium
247
solid
metal
Actinide
7s2 5f8 6d1
1.4-1.3
7
n/a
98
Cf
Californium
251
solid
metal
Actinide
7s2 5f10
1.4-1.3
7
n/a
99
Es
Einsteinium
252
solid
metal
Actinide
7s2 5f11
1.4-1.3
7
n/a
100
Fm
Fermium
257
solid
metal
Actinide
7s2 5f12
1.4-1.3
7
n/a
101
Md
Mendelevium
258
solid
metal
Actinide
7s2 5f13
1.4-1.3
7
n/a
102
No
Nobelium
259
solid
metal
Actinide
7s2 5f14
1.4-1.3
7
n/a
103
Lr
Lawrencium
262
solid
metal
Actinide
7s2 5f14 6d1
–
7
4
104
Rf
Rutherfordium
261
solid
metal
Transition metal
7s2 5f14 6d2
–
7
5
105
Db
Dubnium
268
solid
metal
Transition metal
7s2 5f14 6d3
–
7
6
106
Sg
Seaborgium
269
solid
metal
Transition metal
7s2 5f14 6d4
–
7
7
107
Bh
Bohrium
270
solid
metal
Transition metal
7s2 5f14 6d5
–
7
8
108
Hs
Hassium
269
solid
metal
Transition metal
7s2 5f14 6d6
–
7
9
109
Mt
Meitnerium
277
unknown
unknown
Transition metal
7s2 5f14 6d7
–
7
10
110
Ds
Darmstadtium
281
unknown
unknown
Transition metal
7s2 5f14 6d8
–
7
11
111
Rg
Roentgenium
281
unknown
unknown
Transition metal
7s2 5f14 6d9
–
7
12
112
Cn
Copernicium
285
solid
metal
Transition metal
7s2 5f14 6d10
–
7
13
113
Nh
Nihonium
286
unknown
unknown
–
–
7
14
114
Fl
Flerovium
289
solid
metal
–
–
7
15
115
Mc
Moscovium
288
unknown
unknown
Pnictogen
–
7
16
116
Lv
Livermorium
293
unknown
unknown
Chalcogen
–
7
17
117
Ts
Tennessine
294
unknown
unknown
Halogen
–
7
18
118
Og
Oganesson
294
unknown
unknown
–
–
Links & Resources
Watch The Periodic Table: Crash Course Chemistry #4 (11:21 min)
Watch The Periodic Table Explained (3:06 min)
For suggestions on accessible periodic tables for those with low/no vision, visit:
Low vision and braille versions of the periodic table are available.
Attribution & References
Figure 3 from “3.6 The Periodic Table “, Figure 2 from “3.7 Molecular and Ionic Compounds “, Figure 4 from “3.4 Electronic Structure of the Atoms (General configurations) “, Figure 3 from “4.2 Covalent Bonding ” In General Chemistry 1 & 2 Chemistry (Open Stax) CC BY 4.0 . Access for free at Chemistry (OpenStax)
Figure 11.3c by Revathi Mahadevan from Enhanced Introductory College Chemistry CC BY 4.0
References
Injosoft. (2023). List of chemical elements – periodic table
Periodic Table of the elements