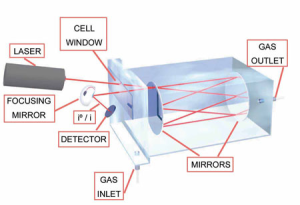
Định luật Beer-Lambert trong quang học là một mối quan hệ thực nghiệm liên hệ sự hấp thụ ánh sáng của một vật liệu với các đặc tính của vật liệu mà ánh sáng truyền qua. Đây là cơ sở của phương pháp quang phổ hấp thụ, cho phép đo nồng độ của các loại khí khác nhau trong một buồng chứa.
Nếu bình chứa có một hỗn hợp gồm [latex]n[/latex] khí ‘‘tinh khiết’’, định luật này giả định rằng logarit của tỷ số cường độ ánh sáng là một hàm tuyến tính của nồng độ của mỗi khí trong hỗn hợp. Do đó, log-tỷ số của các cường độ có dạng [latex]y=a^Tx[/latex] với một véctơ nào đó [latex]a \in \mathbb{R}^n[/latex], trong đó [latex]x[/latex] là véctơ nồng độ. Các hệ số [latex]a_j[/latex], [latex]j= 1, \cdots, n[/latex] tương ứng với log-tỷ số của cường độ ánh sáng khi [latex]x= e_j[/latex] (véctơ thứ [latex]j[/latex] của cơ sở chính tắc, tương ứng với khí tinh khiết thứ [latex]j[/latex]). Đại lượng [latex]a_j[/latex] được gọi là hệ số hấp thụ của khí thứ [latex]j[/latex] và có thể được đo trong phòng thí nghiệm.
Xem thêm: Quang phổ hấp thụ: sử dụng các phép đo ở các tần số ánh sáng khác nhau.