Experiment #3: Brownian motion
3. What to do after the lab?
3.1 Data Analysis
To characterize the motion of the beads captured in the movies you obtained, use the MosaicSuite ImageJ plugin to track the beads.
- Install MosaicSuite according to the instructions given on the Mosaic website: http:// mosaic.mpi-cbg.de/?q=downloads/imageJ. Please see the tutorial in this book called Fiji/ImageJ2 Tutorials.
- Before you start analyzing real movies of beads, analyze the motion of the simulated beads in the movie “BrownianMotionSimulation.avi”, following the instructions in #Using MosaicSuite (Fiji/ImageJ2 Tutorials). It is very important that you complete this step, in order to get familiar with the particle tracker software (but you do not need to discuss it in your lab report).
- Open the movie you want to analyze in ImageJ (File -> Open). Chose “Convert to Grayscale”. Do not use a virtual stack.
- Measure the apparent size of the beads in pixel.
- Prepare your video for analysis by doing the following:
- If possible, reduce the movie size to allow for easier and faster particle tracking. You can do so by choosing a region of interest with the “Rectangle” tool (for example a small region containing one or several visible mobile particles) and then cropping the video (Image -> Crop). In addition, if only part of the movie is of interest (for example, if the bead you wish to track is in focus only for part of the movie), you should consider reducing the number of frames to keep only the interesting part (using the tool Image -> Stacks -> Tools -> Make Substack).
- If necessary, invert the intensity so that your particles appear as white objects on a dark background (instead of black on a grey background), by using Edit -> Invert.
- Adjusting the brightness and contrast of the image in order to obtain the clearest possible image (Image -> Adjust -> Brightness/Contrast -> Auto).
- Adjust the image properties by going to Image -> Properties. Remember to:
- Set the number of slices to 1 and the number of frames to the actual number of frames in the movie.
- Enter the correct frame interval (which you can get by dividing the movie acquisition time by the number of frames, or checking what is the time difference between the time stamps of consecutive frames, as shown at the top of the window showing your movie).
- Enter the correct pixel width in micron (enter “um” as the unit of length). Use an image of a calibration slide and the Analyze -> Set Scale function to obtain the pixel width (if you tick the “Global” box in the Set Scale tool while your movie is open, the scaling will apply to all open movies and images and the value of the pixel width will be entered automatically in the Properties of your movie).
- Use the tracking plug-in (Plugins -> Mosaic -> Particle tracker 2D/3D) to detect the beads’ trajectories.
- Adjust the value of parameters “Radius”, “Cutoff” and “Percentile” as explained in Appendix 1, until you see that particles are detected throughout most of the movie (depending on the quality of the movie, it might not be possible to achieve perfect detection in all frames).
- Once you are happy with the detection, set the values of “Link Range” (3 is a good start) and “Displacement”, then click “Ok”. Visualize the detected trajectories (Visualize all trajectories). The motion of a bead might be broken up into several trajectories, which can be fixed if necessary by increasing the link range and/or the displacement. If you are happy with the trajectories, you can save them in a table (“All trajectories to table”).
- You can select a particular trajectory by selecting first “Visualize all trajectories”, then clicking on the desired trajectory. Once a trajectory is selected, you can press “MSS/MSD plots” then “MSD” in order to obtain the particle MSD and diffusion coefficient.
- Are your results consistent with those obtained by Jean Perrin? Can you estimate Avogadro’s number from the bead’s diffusion coefficient?
Note: The viscosity of water at room temperature is ![]() =1×10−3 kg m–1 s-1.
=1×10−3 kg m–1 s-1.
3.2 Lab Report
- Write a short (3 pages maximum including figures and references) “lab report” containing:
- a very short introduction about Brownian motion
- a very short method section giving some details on the movies that you analyzed and on the analysis methods you employed.
- a result section with figures showing some trajectories, some MSDs, and the result of your diffusion coefficient measurement (for example a plot showing
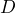 as a function of
as a function of 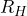 ). You can also include a table with the values of
). You can also include a table with the values of 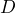 you measured. Include an estimate of Avogadro’s number. Don’t forget errors and significant digits when reporting on your results.
you measured. Include an estimate of Avogadro’s number. Don’t forget errors and significant digits when reporting on your results. - a short discussion section where you comment on whether the beads you tracked behave as Brownian particles and where you compare your estimate of Avogadro’s number with its known value.
- Upload your lab report on Crowdmark.
Lab 3 Notes/FAQs
- Fill and seal one channel at a time! Otherwise they may dry out by the time you get to a second channel.
- Record your bead sizes clearly!
